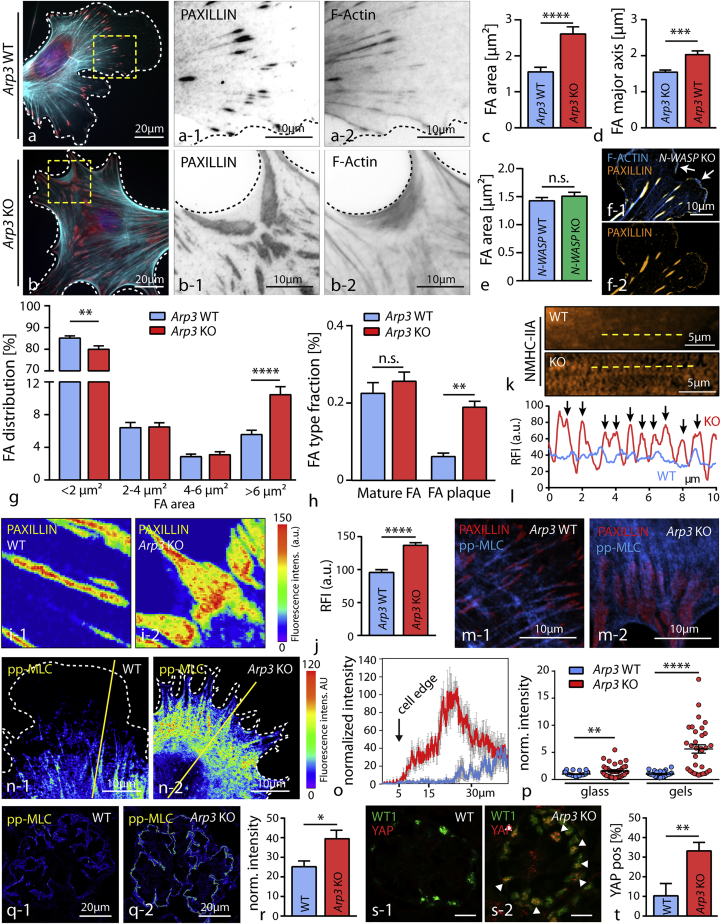
Figure 4.
Loss of ARP3 Results in Altered Focal Adhesion Morphology and Increased Actomyosin Activity
(A and B) Staining for the focal adhesion (FA) component PAXILLIN (red) revealed confluent FA clusters in primary Arp3 KO podocytes (B-1), which were connected via filamentous actin webs (wild type (A) and KO (B)). F-actin (blue) was stained by Phalloidin.
(C and D) Quantification of average focal adhesion area (C) and major axis (D) showed increased values for both parameters in respective KO cells (25 wild-type and 28 Arp3 KO cells were analyzed; ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001).
(E and F) Average focal adhesion area (E) and lamellipodium formation (F) was not obviously impaired in primary N-WASP KO podocytes in 2D culture conditions (n = 36 cells per genotype were analyzed, n.s., non significant; FA were stained by PAXILLIN and F-actin by phalloidin; white arrows indicate lamellipodia).
(G) Analysis of focal adhesion clusters due to their individual size indicated a shift toward enlarged adhesion clusters in Arp3 KO podocytes (25 WT and 26 Arp3 KO cells were analyzed; ∗∗p < 0.01, ∗∗∗∗p < 0.0001).
(H) This shift in FA size was paralleled by a change in the distribution of individual FA classes: loss of Arp3 resulted in the accumulation of FA plaques (n = 18 cells per genotype were analyzed; n.s., non significant, ∗∗p < 0.01).
(I and J) Analysis of PAXILLIN fluorescence intensities (FI) in wild type podocytes (I-1) and in respective Arp3 KO cells (I-2) revealed altered intensity patterns and increased mean PAXILLIN intensity (J) per focal adhesion (n = 100 mature FA per genotype were analyzed; ∗∗∗∗p < 0.0001).
(K and L) Immunofluorescence for NMHC-IIA (K) demonstrated an increased sarcomere-like pattern of actin fibers (L) in respective Arp3 KO podocytes (yellow lines indicate positions for line scan measurements, and black arrows indicate myosin-2 peaks).
(M) Immunofluorescence staining for pp-MLC and the focal adhesion marker PAXILLIN illustrated prominent, fiber-like accumulations of pp-MLC linked to FAs in Arp3 KO podocytes.
(N) Staining for pp-MLC revealed increased MLC activation levels in Arp3 KO podocytes, condensed in arc regions between pseudopodial protrusions. Also a ring-like distribution was observed in the majority of Arp3 KO podocytes. (Yellow lines indicate representative positions for line scan measurements).
(O) Line scan profiles for pp-MLC showed highest activation levels close to the cell edge in Arp3 KO cells (values represent mean intensities of 6 cells per genotype; gray error bars represent SEM).
(P) Quantification of fluorescence intensity for pp-MLC on either glass or soft gels revealed higher MLC activation levels for Arp3 KO podocytes (37 wild-type and 41 Arp3 KO cells on glass; 31 wild-type and 32 Arp3 KO cells on gels were analyzed; ∗∗p < 0.01, ∗∗∗∗p < 0.0001).
(Q and R) Staining for pp-MLC in glomeruli from either wild-type (Q-1) or Arp3 KO (Q-2) animals showed high levels of phosphorylated MLC in the podocyte compartment, as visualized with the podocyte-specific marker NEPHRIN (mean podocyte pp-MLC intensity of n = 4 WT and 4 KO animals were statistically analyzed; ∗p < 0.05; the mean pp-MLC intensity per animal was calculated from mean pp-MLC intensity of the segmented podocyte compartment of at least 20 glomeruli per animal).
(S and T) Immunofluorescence staining for the mechanical tension marker YAP in glomeruli from either wild-type or Arp3 KO (S) animals showed increased percentages of YAP-positive podocyte nuclei in KO animals (T). Podocyte nuclei were visualized by the podocyte-specific marker WT1 (the mean percentage of YAP-positive podocyte nuclei per glomerulus of n = 5 WT and 5 KO animals were analyzed; white arrow bars indicate podocyte nuclei with co-occurring positivity for YAP, ∗∗p < 0.01; the percentage of YAP positive podocyte nuclei per glomerulus per animal was calculated from at least 20 glomeruli per animal). All data are represented as mean ± SEM.