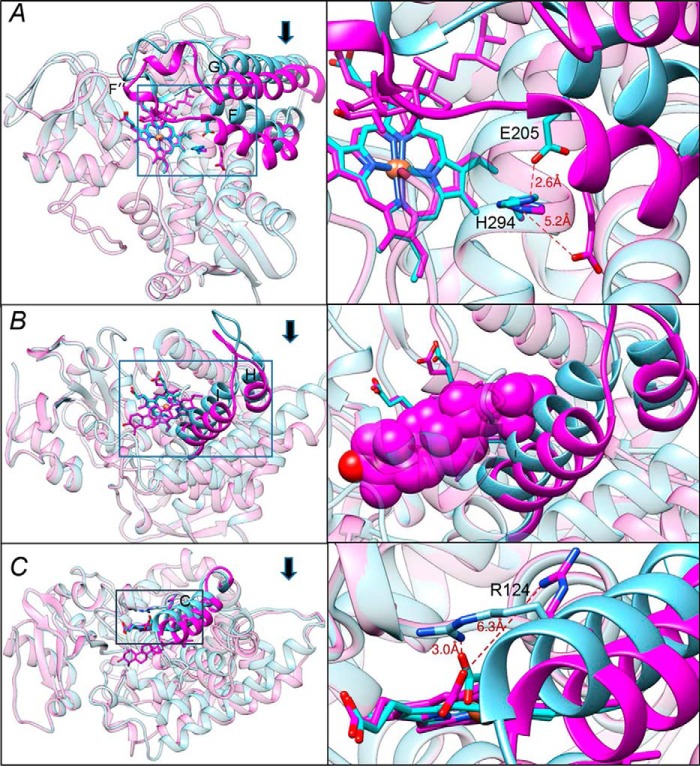
Figure 3.
Conformational switch in the CYP51 structure upon binding of the substrate. The three segments of the active site that experience large-scale conformational rearrangements are shown in cyan (ligand-free structure) and magenta (substrate-bound structure). Arrows show the direction of movement. The other portions of the molecules are presented as semitransparent ribbons of the corresponding colors. Left panels, the overall view of the superimposed structures (see also Fig. S6); right panels, the enlarged view of the squared fragments. The structures were superimposed in LSQKAB (CCP4 Suite). A, the FG arm and the conserved histidine-acid salt-bridge opening (activation of proton relay machinery). B, the HI segment. In the enlarged fragment, obtusifoliol is presented as van der Waals spheres to show that without the movement it would collide with the (cyan) I helix. C, helix C. The 3.5–4 Å movement of this helix reshapes the CYP51 proximal surface. The inset shows rearrangements in the position of Arg-124, whose guanidino group now protrudes above the CYP51 proximal surface, bringing additional positive charge to its electrostatic potential (shown in Fig. 5).