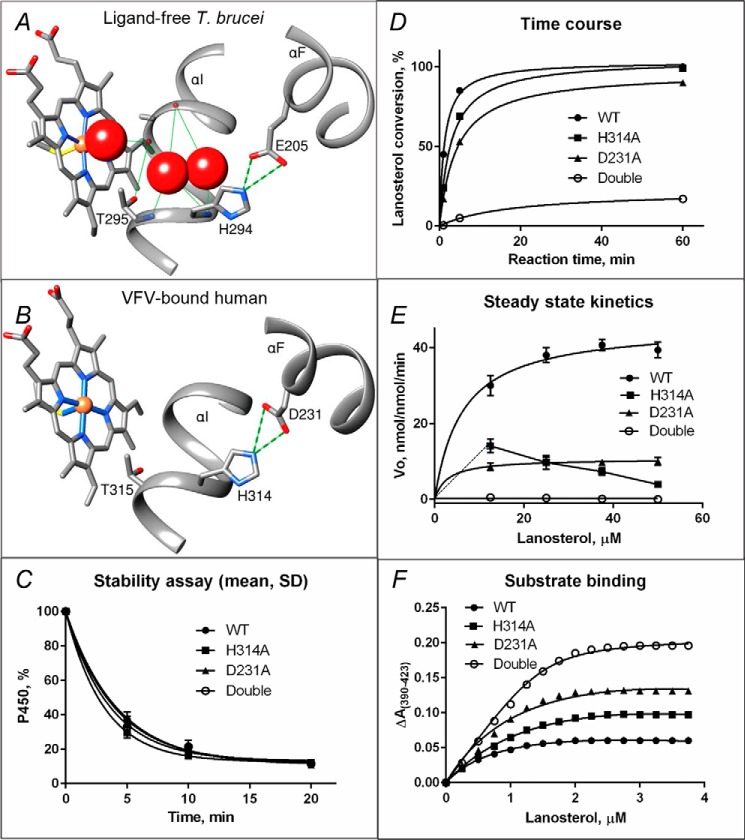
Figure 4.
Proposed proton delivery route in the CYP51 family. A, ligand-free water-coordinated protozoan CYP51 (PDB code 3G1Q, 1.8 Å resolution). The water molecules are presented as red spheres. B, inhibitor-bound human CYP51 (PDB code 4UHI, 2.0 Å resolution). C–F, mutants of the His-acid salt bridge in human CYP51. C, stability at 42 °C monitored as the decrease in the P450 content, the initial P450 concentration was 2 μm (see also Fig. S7). D, time course of substrate conversion, 0.5 μm P450, 25 μm lanosterol. E, Michaelis–Menten plots using 0.25 μm P450, 1 min reaction; points are shown as means of three determinations ± S.D. F, spectral response to lanosterol; 2 μm P450 (fit to Morrison equation, nonlinear regression). The estimated low-to-high spin transition was 30, 50, 68, and 94% in the WT, H314A, D231A, and the H314A/D231A double mutant proteins, respectively (see also Table 2).