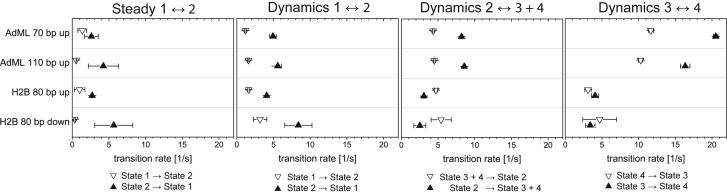
Figure 7.
Comparison of the kinetics for the different constructs. Resulting rate constants of the four different sample preparations are shown. (k1 ↔ 2) The rate constants for transitions to a possible alternative TBP-binding site (EFRET = 0.20) in the absence (left panel) and presence (middle left panel) of NC2 are shown. The transition rate from EFRET = 0.40 to 0.20 is between 2 and 8 s−1 (arrow up) and the return the rate lies between 0.5 and 3.5 s−1 (arrow down). The affinity to this binding site appears to be neither dependent on the promoter nor influenced by the addition of NC2. (k2 ↔ 3 + 4) The rate for transitions between the EFRET = 0.40 and higher FRET states are shown (middle right panel). The transition rate from the bent conformation to the higher FRET conformations (arrow up) is a factor of ~3 higher for the AdML promoter than for the H2B promoter, whereas the relaxation rate for returning to the bent 40% FRET conformation (arrow down) was similar for all four samples. (k3 ↔ 4) The transition rates between the intermediate and high FRET states are shown. The transition rates from the intermediate state to high FRET conformation (arrow up) and back (arrow down) were faster for the AdML promoter than for the H2B promoter. The error bars are the standard error of the mean (SEM) determined empirically by splitting the data set into four equal subsets (two subsets in the case of transitions in the absence of NC2 due to limited statistics) and calculating the mean and the standard error of the mean (SEM) of the different subsets.