Short abstract
Alemtuzumab is a high-efficacy disease-modifying therapy for the treatment of relapsing forms of multiple sclerosis and is associated with secondary autoimmune adverse events. We report a novel case of secondary autoimmune myositis that occurred seven months after the initial treatment cycle and achieved full recovery with oral corticosteroids. This particular form of myositis appears to be unique, and is likely to be a distinct entity from the other four types of immune-mediated myositis.
Keywords: Alemtuzumab, immunology, multiple sclerosis, disease-modifying therapies
Introduction
Alemtuzumab is a humanized monoclonal antibody that targets CD-52 resulting in prolonged lymphocyte depletion and is an effective disease-modifying therapy for relapsing–remitting multiple sclerosis (MS).1 Patients treated with alemtuzumab have a 30% chance of acquiring secondary autoimmunity, most frequently affecting the thyroid, followed by blood abnormalities such as immune thrombocytopenia. Rarely, patients may develop an autoimmune glomerulopathy affecting the basement membrane resulting in acute kidney injury.2 We report the first case of autoimmune myositis in a 44-year-old female treated with alemtuzumab.
Case report
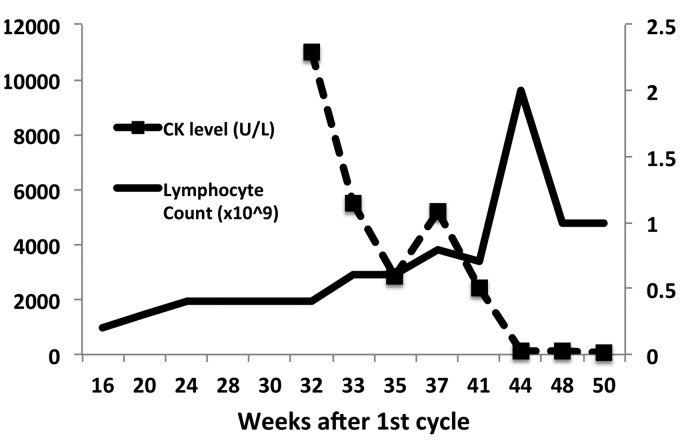
In 2003, at age 31 years, our patient experienced lower back and paresthesia that spontaneously resolved. Over the subsequent years she developed sensory symptoms in her lower limbs and magnetic resonance imaging (MRI) confirmed a diagnosis of MS at age 34 years. The patient was initially treated with interferon-beta 1b before changing to glatiramer acetate. The disease progressed clinically and radiologically, with lesions distributed throughout the deep white matter, optic nerves and spinal cord and she was treated with natalizumab followed by fingolimod due to an increased risk of progressive multifocal leukoencephalopathy. Ultimately, she received her first cycle of alemtuzumab in November 2015 with a baseline Expanded Disability Status Scale (EDSS) level of 1.5. The patient did not experience any significant adverse events throughout the first six months after the induction therapy and by early June of 2016 had a lymphocyte count of 0.4 × 109/l (Figure 1). Soon after this she developed myalgia affecting both arms and legs in a predominantly proximal distribution. MRI scans did not reveal any progression of MS, but blood investigations showed a grossly elevated creatine phosphokinase (CK) level of 11,115 U/l (<211 U/l). There was also elevation of her hepatic transaminases, AST 322 U/l (12–36 U/l) and ALT 129 U/l (<55 U/l). The ANA, ENA, ANCA, rheumatoid factor and anti-CCP antibodies were negative. The following autoimmune myositis markers were also negative – Ro-52, OJ, EJ, PL-12, PL-7, SRP, Jo-1, PM-Scl 75, PM-Scl 100, Ku, Mi-2 (Myositis Profile Euroline Blot, Euroimmun, Lübeck, Germany) and HMGCR (in house ELISA, PathWest Laboratory Medicine, Western Australia). Viral serology was negative for acute infection including VZV, EBV, CMV and nasal swabs were negative for influenza. The patient was hepatitis B, C and HIV negative.
Figure 1.
Creatine-phosphokinase level and lymphocyte count after first cycle of alemtuzumab.
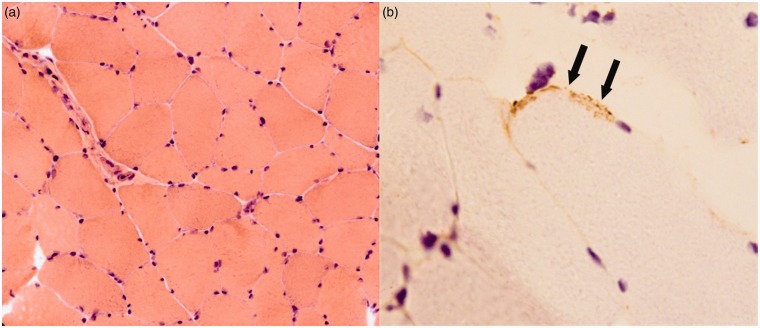
Electromyography did demonstrate myopathic units in keeping with a mild generalized non-necrotizing process. MRI scans of the right deltoid and both quadriceps did not show any inflammation. A muscle biopsy obtained from the left quadriceps demonstrated a few small clusters of lymphocytes without evidence of myonecrosis. The terminal components of complement (C5b9) were identified in some muscle fibers (Figure 2). Staining for MHC-1 and immunoglobulin (Ig)G was negative and electron microscopy did not demonstrate any viral changes.
Figure 2.
Quadriceps muscle biopsy demonstrating minimal inflammatory infiltrate and no evidence of necrosis, swelling or atrophy (a). Occasional muscle fiber membranes stained positively for C5b9, the terminal component of complement (arrows, (b)). (a): Haematoxylin and Eosin (H&E)×20; (b): C5b9 × 40.
Corticosteroid therapy was started immediately after the muscle biopsy excluded intramuscular infection at a dose of 50 mg orally per day, and this was slowly weaned to a dose of 10 mg over a three-month period. The patient’s symptoms resolved soon after the commencement of treatment and this correlated with decreases in serum CK levels (Figure 1). Overall the patient has improved neurologically and her current EDSS level is 1. There were no sequelae of myositis.
Discussion
This is the first reported case of myositis secondary to the use of alemtuzumab. We propose that this is an autoimmune myopathy based on the timing of the initial symptoms after alemtuzumab exposure, the rapid lymphocyte reconstitution at that time, and exclusion of an infective pathology. Clinically the duration of symptoms and persistent CK level for eight weeks, with complete resolution after corticosteroid therapy make a viral etiology unlikely.3 The rapid response to corticosteroid therapy has also been observed with alemtuzumab associated ITP.4
The pathogenesis of the elevated CK and the classification of this myopathy within the spectrum of inflammatory muscle diseases warrants study and discussion. The leakage of CK was large with only minor changes present on EMG and muscle biopsy. This implies that necrosis of muscle fibers did not occur but disruption to the muscle membrane could have been widespread. Certainly, the patient’s symptoms were consistent with this explanation, characterized by generalized muscle pain, without weakness. In the context of a depleted peripheral lymphocyte count it is not surprising that there was minimal lymphocytic infiltrate in the muscle biopsy, but this further adds to the complexity in understanding how the damage occurred. Although we could not demonstrate IgG binding to muscle, the terminal components of complement (C5b9) were identified in some muscle fibers implying that humoral mechanisms were involved. Following alemtuzumab, B-lymphocytes reconstitute more rapidly than T-lymphocytes. Importantly, regulatory T-cell repopulation occurs more slowly than that observed for all B-cells and therefore may not inhibit reconstituted B-cell-mediated autoimmunity.5
The four main categories of inflammatory myopathy include dermatomyositis, polymyositis, necrotizing autoimmune myositis, and inclusion-body myositis, with each having distinct histopathological features.6 Alemtuzumab-associated myositis is likely to be a distinct entity from these four conventional forms of myositis. There is a lack of rash and paucity of lymphocytic infiltration that differentiates this disease from dermatomyositis and polymyositis. It also lacks necrosis and the auto-antibody associations observed in necrotizing autoimmune myositis and inclusion-body myositis.
With increased international use of alemtuzumab for MS it is highly likely that further secondary idiosyncratic immune-mediated adverse events will occur in these patients.7 We encourage the reporting of such events in order to assist clinicians in the recognition and management of such diseases.
Acknowledgements
The authors would like to thank the patient for consenting to the publication of this clinical information.
Conflict of Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: P Aouad has received travel funding and speaker honoraria from Sanofi Genzyme, Merck, Biogen, Teva and Roche pharmaceuticals and served on the MS global advisory network for Merck. C Yiannikas has received travel funding from Sanofi Genzyme and Ipsen and consulted for Allergan and Ipsen. J Parratt has served on the scientific advisory board of Sanofi Genzyme, Novartis, and Biogen; received speaker honoraria from Biogen, Genzyme/Sanofi, and Merck Serono; received research support from Multiple Sclerosis Research Australia, Neil and Norma Hill Inaugural Junior Practitioner Fellowship.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Coles AJ, Twyman CL, Arnold DL, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: A randomised controlled phase 3 trial. Lancet 2012; 380: 1829–1839. [DOI] [PubMed] [Google Scholar]
- 2.Costelloe L, Jones J, Coles A. Secondary autoimmune diseases following alemtuzumab therapy for multiple sclerosis. Expert Rev Neurother 2012; 12: 335–341. [DOI] [PubMed] [Google Scholar]
- 3.Crum-Cianflone NF. Bacterial, fungal, parasitic, and viral myositis. Clin Microbiol Rev 2008; 21: 473–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cuker A, Coles AJ, Sullivan H, et al. A distinctive form of immune thrombocytopenia in a phase 2 study of alemtuzumab for the treatment of relapsing–remitting multiple sclerosis. Blood 2011; 118: 6299–6305. [DOI] [PubMed] [Google Scholar]
- 5.Baker D, Herrod SS, Alvarez-Gonzalez C, et al. Interpreting lymphocyte reconstitution data from the pivotal phase 3 trials of alemtuzumab. JAMA Neurol 2017; 74: 961–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dalakas MC. Inflammatory muscle diseases. N Engl J Med 2015; 372: 1734–1747. [DOI] [PubMed] [Google Scholar]
- 7.Berger T, Elovaara I, Fredrikson S, et al. Alemtuzumab use in clinical practice: Recommendations from European multiple sclerosis experts. CNS Drugs 2017; 31: 33–50. [DOI] [PMC free article] [PubMed] [Google Scholar]