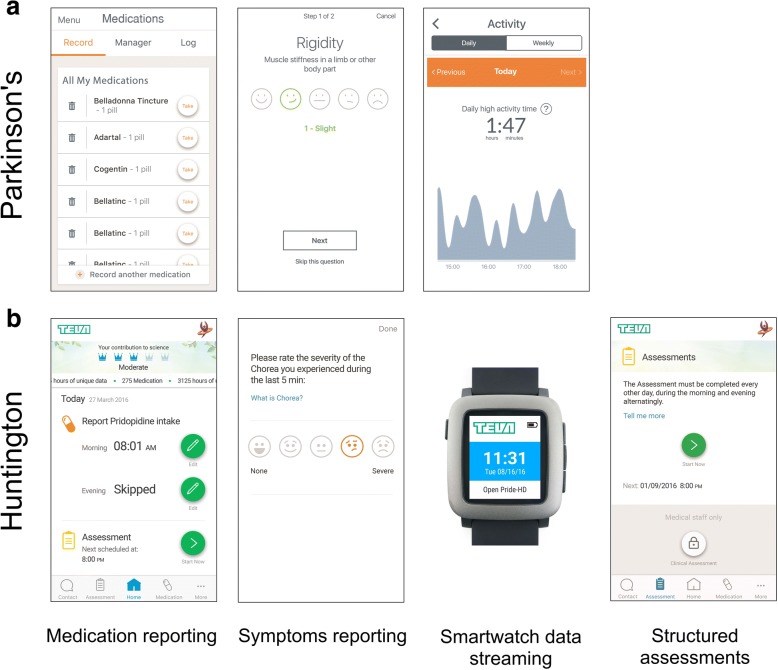
Fig. 1.
The four remote protocol compliance metrics tracked in this report using the Intel® Pharma Analytics Platform with their associated smartphone application screenshots. a The PD trial tracked three metrics as defined by the study protocol: app-based medication reporting of the patient’s normal, predefined medication regimen, smartwatch data streaming excluding charging time, and app-based daily symptoms reporting. b The HD trial tracked four compliance measures as defined by the study protocol: app-based medication reporting of the investigational drug, daily smartwatch data streaming excluding charging time, app-based daily symptoms reporting, and performance of structured motor assessments at home. The smartwatch graphic was obtained from Wikimedia Commons