Abstract
Background and objectives
High-quality epidemiologic data on AKI in children are particularly lacking in developing countries. This study aimed to assess the epidemiology and clinical correlates of AKI among hospitalized children in China.
Design, setting, participants, & measurements
We performed a multicenter study, in a cohort of hospitalized children aged 1 month to 18 years, from 25 general and children’s hospitals in China during 2013–2015. We obtained patient-level data from the electronic hospitalization information system and laboratory databases of all children who had at least two serum creatinine tests within any 7-day window during their first 30 days of hospitalization. We identified AKI events according to the creatinine criteria of Kidney Disease Improving Global Outcomes. The in-hospital outcomes of AKI, including mortality, kidney recovery, and length of stay, were assessed. We estimated the corresponding hazard ratios using a Cox proportional hazard model, with adjustment for age, sex, comorbidities, and clinical procedures.
Results
A total of 19,908 (20%) patients with AKI were identified among 101,836 pediatric inpatients, of which 7220 (7%) were community acquired and 12,688 (13%) were hospital acquired. Up to 96% of these AKI events were not diagnosed on the discharge records. The cumulative incidence of AKI in infants (28%) was twice that in adolescents (12%). The profiles of risk factors differed between community-acquired and hospital-acquired AKI and varied with age. Diarrhea and sepsis were the top risk factors for community-acquired AKI, each contributing 6% of the risk. Congenital heart disease/cardiac surgery was the major risk factor for hospital-acquired AKI, contributing to 19% of cases. Exposure to nephrotoxic drugs, mostly nonsteroidal anti-inflammatory drugs and proton pump inhibitors, was common in hospitalized children and was associated with a higher risk of AKI. Death occurred in 842 out of 19,908 patients (4%) with AKI versus 450 out of 81,478 children (0.5%) without AKI. The risk of in-hospital death was higher among children with severe AKI, shock, and respiratory failure. Pediatric AKI was associated with longer hospital stay and higher daily cost, even after adjustment for covariates.
Conclusions
Pediatric AKI is common and is substantially underdiagnosed in China.
Keywords: acute renal failure, children, clinical epidemiology
Visual Abstract
AKI is frequent in high-risk hospitalized children and is associated with higher mortality and sequelae that lead to CKD in adult life (1–9). Worldwide epidemiologic data on AKI in children are limited because of the fact that most of the studies on pediatric AKI are from developed countries (5,10–17). High-quality data from the developing world, including most Asian countries, are particularly scarce (18,19). Moreover, previous epidemiologic studies on pediatric AKI were usually limited to critically ill patients or subgroups with specific clinical settings, such as cardiac surgery, nephrotic syndrome, or nephrotoxic exposure (20–26).
China is the largest developing country, with 20% of the world population. However, most epidemiologic data on pediatric AKI in China are gleaned from single-center studies with small sample sizes (19,27–30). The incidences of pediatric AKI reported by these studies (0.31%–1.4%) are substantially lower than those from the developed countries (17.0%–26.9%) (1,31). Notably, most studies from China diagnosed pediatric AKI using International Classification of Diseases (ICD) codes, which have been shown to underestimate the disease burden (32–34). Until now, nationwide epidemiologic study of pediatric AKI has not been available in China.
Here, we conducted a large, multicenter study in a large cohort of hospitalized children from 25 general and children’s hospitals across China, encompassing a wide range of disease spectrums and severities. The aims of the study were to estimate the incidence of pediatric AKI, to describe the profile of risk factors for both hospital-acquired and community-acquired AKI in children, and to evaluate the association of pediatric AKI with in-hospital outcomes in China.
Materials and Methods
Study Design, Population, and Data Source
The Epidemiology of AKI in Chinese Hospitalized Patients 2 (EACH2) study is a multicenter cohort study. We invited 15 children’s hospitals and 17 general hospitals in 16 provinces across China to participate in the study. A total of 25 medical centers comprising nine of the 15 children’s hospitals and 16 of the 17 general hospitals, spanning 15 provinces, agreed to participate. The list of participating hospitals is provided in the Supplemental Appendix. The study cohort included 3,044,224 patients admitted to a participating hospital from January 1, 2013 to December 31, 2015. The analysis set comprised 101,836 pediatric inpatients aged between 1 month and 18 years, who had at least two serum creatinine tests within any 7-day window during their first 30 days of hospitalization (Supplemental Figure 1). We only included creatinine tests that used an enzymatic assay, and excluded patients diagnosed with ESKD or receiving maintenance dialysis or kidney transplantation. For patients with multiple hospitalizations, we only included the first hospitalization in the analysis set.
The study centers were asked to export the hospitalization record and the laboratory and prescription data of all hospitalized patients within the study period from the electronic hospital information system. The hospitalization records consisted of patients’ age, sex, date of admission, diagnosis code at admission and discharge, surgical procedures and dates, need for intensive care, in-hospital death, and total hospitalization cost. The laboratory data included the value and time of patients’ serum creatinine tests. The prescription data, which included the type of drug or procedures prescribed, dose, and start and stop time, were available in 50,989 (50%) inpatients in the analysis set. The exported data from all study centers were pooled and cleaned at the National Clinical Research Center for Kidney Disease in Guangzhou. The Medical Ethics Committee of Nanfang Hospital approved the study protocol.
Identification and Classification of AKI
AKI was defined as an increase in serum creatinine by 0.3 mg/dl within 48 hours or a 50% increase in serum creatinine from the baseline within 7 days, according to the Kidney Disease Improving Global Outcomes (KDIGO) criteria (35). We screened patients’ serum creatinine for the onset of AKI using an algorithm described previously (36). In brief, serum creatinine data during hospitalization were sorted in increasing order according to the test time. At any time point, t, a baseline creatinine was dynamically defined as the mean creatinine level within the last 90 days before t, and each of the available creatinine data within 7 days after t was compared with this baseline. The earliest day that the creatinine change met the KDIGO criteria was defined as the date of AKI detection.
Patients who met at least one of the following criteria were classified as having community-acquired AKI (36): (1) admitted with AKI according to diagnosis code; (2) having multiple creatinine tests on or before the first day of hospitalization, and the increase in creatinine on the first day met the KDIGO definition; or (3) serum creatinine on admission was ≥1.5-fold for the age- and sex-standardized serum creatinine reference value (Supplemental Material) and ≥1.5-fold of the minimal serum creatinine level during hospitalization. The baseline creatinine level was calculated as the lowest serum creatinine during hospitalization for patients with community-acquired AKI, and the mean serum creatinine during the first 30 days of hospitalization for those without AKI. Patients that met the KDIGO creatinine criteria but not the criteria for the community-acquired AKI were diagnosed with hospital-acquired AKI.
The stage of AKI was determined using the peak serum creatinine level after AKI detection, with an increase of <100%, ≥100%, and ≥200% over baseline being defined as stage 1, 2, and 3, respectively.
Physician-diagnosed AKI on admission or at discharge were determined according to ICD-10 Clinical Modification codes (N17.051, N17.153, N17.252, N17.851, O90.451).
Outcomes, Comorbidity, and Drug Usage
The primary outcome was the time to in-hospital death. Other outcomes included length of hospital stay (LOS), daily cost of hospitalization, and time to kidney recovery. Hospital-acquired AKI was considered to have achieved kidney recovery if serum creatinine decreased to within “non-AKI” range and was at least 0.3 mg/dl or 33% lower than the peak level in the absence of kidney replacement therapy.
Physician-diagnosed AKI and all coexisting conditions, at admission and discharge, were all determined by the diagnosis codes (ICD10 Clinical Modification codes). Surgical procedures were classified into major cardiac surgeries and noncardiac surgeries.
We classified drugs according to the Anatomic Therapeutic Chemical classification system (37). We defined drug use as any prescription of the specified drug within 1 week before the 7-day serum creatinine test window.
Statistical Analyses
We classified those aged 1 month to 1 year, 2–10 years, and 11–18 years as infant, child, and adolescent, respectively (10). We calculated the frequencies of community-acquired AKI and hospital-acquired AKI that occurred during hospitalization stratified by age groups and clinical settings in the analysis set. We estimated the cumulative incidence rates of hospital-acquired AKI with and without stratification by age group and need for intensive care using the Kaplan–Meier method. Because the majority of the hospitalized children did not have enough serum creatinine measurements to be screened for AKI status, and these patients had fewer risk factors for AKI, the AKI incidence estimated in the analysis set may be higher than that in the total population. Therefore, we used a weighted Kaplan–Meier method under a probability sampling model to extrapolate the AKI incidence estimated in the analysis set to the total hospitalizations (38). In this survey model, we built a logistic regression model for being selected into the analysis set, with adjustment for age, sex, hospital, need for intensive care, comorbidities, and clinical procedures, and the corresponding probability from the regression model was taken as the sampling probability.
We estimate the hazard ratios (HRs) of all possible risk factors for hospital-acquired AKI, including age, sex, baseline serum creatinine, comorbidities, and clinical procedures, using a Cox proportional hazard model stratified by hospital and the need for intensive care. Clinical procedures were coded as time-varying covariates to allow for more accurate estimation of the risk. Similarly, we used a logistic regression model to estimate the odds ratios of the risk factors for community-acquired AKI, without adjustment for clinical procedures. We estimated the association of drug use on the risk of hospital-acquired AKI using a logistic regression model with adjustment for the propensity score of drug use, time from admission, age, sex, center, baseline serum creatinine, comorbidities, clinical procedures, and need for intensive care. We calculated the population attributable fractions (PAFs) of the significant risk factors using the formula 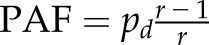 , where r is the estimated relative risk and pd is the proportion of patients with AKI exposed to the risk factor of interest (39).
, where r is the estimated relative risk and pd is the proportion of patients with AKI exposed to the risk factor of interest (39).
We calculated the cumulative rates of in-hospital death in the subgroups by AKI status using the Kaplan–Meier method, and estimated the corresponding HRs using a Cox proportional hazard model with adjustment for age, sex, comorbidities, and clinical procedures, and stratified by hospital and need for intensive care. We used the Kaplan–Meier method to estimate the cumulative rate of kidney recovery after onset of hospital-acquired AKI and the median time to recovery among patients with different AKI stages. We also compared the association of AKI on average daily cost during hospitalization and LOS under a linear regression model, with log transformation of the response variable and adjustment for age, sex, center, comorbidities and clinical procedures.
We performed all statistical analyses using R version 3.3.1, the “survival” package version 2.39–4, and the “survey” package version 3.31–5.
Results
Of 947,854 children aged 1 month to 18 years, 84% had none (15%) or only one (69%) serum creatinine test during hospitalization. We analyzed a total of 101,836 hospitalizations with two or more serum creatinine tests in a 7-day window within the first 30 days of hospitalization. The characteristics of the children included in and excluded from the analysis set are summarized in Supplemental Table 1. Only 6.8% of hospitalized children had serum creatinine data within 90 days before hospitalization. The proportion of patients requiring intensive care and in-hospital mortality were significantly higher in the analysis set than that in the excluded population. We identified a total of 12,688 incidences of hospital-acquired AKI and 7220 incidences of community-acquired AKI in the analysis set. Among children with AKI, 4% received acute dialysis. The characteristics of the children in the analysis set stratified by AKI subtype are summarized in Table 1.
Table 1.
Characteristics of the study population
| Characteristic | Non-AKI, n=81,928 | Community-acquired AKI, n=7220 | Hospital-acquired AKI, n=12,688 |
|---|---|---|---|
| Age, yr | 5 (5.2) | 4 (4.7) | 3 (4.0) |
| Infancy, 1 mo to 1 yr | 23,748 (29) | 2974 (41) | 6082 (48) |
| Childhood, 2–10 yr | 41,911 (51) | 3196 (44) | 5493 (43) |
| Adolescence, 11–18 yr | 16,269 (20) | 1050 (15) | 1113 (9) |
| Sex | |||
| Men | 49,572 (61) | 4492 (62) | 7501 (59) |
| Women | 32,356 (39) | 2728 (38) | 5187 (41) |
| Location | |||
| Central | 36,678 (45) | 3751 (52) | 6752 (53) |
| Northern | 23,461 (29) | 1900 (26) | 2425 (19) |
| Southern | 21,789 (27) | 1569 (22) | 3511 (28) |
| Hospital type | |||
| Children’s hospital | 49,508 (60) | 4561 (63) | 9055 (71) |
| General hospital | 32,420 (40) | 2659 (37) | 3633 (29) |
| Number of creatinine tests | 2 (2, 4) | 4 (3, 6) | 3 (2, 5) |
| Having creatinine data within 90 d before hospitalization | 5750 (7) | 349 (5) | 865 (7) |
| Baseline creatinine, mg/dl | 0.32 (0.25, 0.44) | 0.28 (0.22, 0.40) | 0.24 (0.18, 0.32) |
| AKI stage | |||
| Stage 1 | — | 2263 (31) | 6845 (54) |
| Stage 2 | — | 2259 (31) | 3230 (25) |
| Stage 3 | — | 2698 (38) | 2613 (21) |
| In-hospital death | 450 (0.5) | 168 (2) | 674 (5) |
| Need intensive care | 9728 (12) | 2332 (32) | 2806 (22) |
| Length of stay, d | 13 (9, 21) | 16 (11, 27) | 16 (11, 26) |
Age is expressed as mean (SD); baseline creatinine, number of creatinine test, having creatinine data within 90 days before hospitalization, length of stay, and daily cost are expressed as median (25th, 75th quartiles); other data are expressed as N (%). —, not applicable.
Incidence and Physician Diagnosis Rate of AKI
Community-acquired AKI and hospital-acquired AKI occurred in 7% and 13% of hospitalizations in the analysis set, respectively, giving rise to an overall incidence of 20%. The cumulative incidence rates of community-acquired AKI and hospital-acquired AKI in the total population (including those without serum creatinine measurement) were estimated at 5% and 10%, respectively, under a probability sampling model.
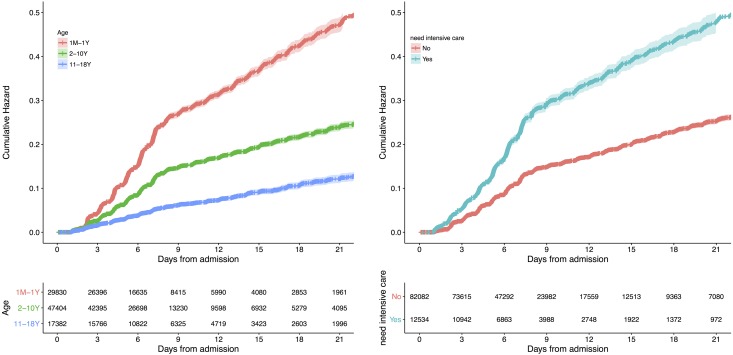
Hospital-acquired AKI occurred more frequently during the first week of hospitalization (Figure 1). The cumulative incidence of hospital-acquired AKI in the analysis set was 12%, 19%, 25%, and 29% on day 7, 14, 21, and 28, respectively. The incidence of AKI stratified by age, type of hospital, and geographic location is presented in Supplemental Table 2.
Figure 1.
The cumulative incidence of hospital acquired AKI was higher with increasing number of days from admission and were higher in children with younger age and those need for intensive care.
Among the patients with AKI identified by creatinine measurements, only 4% were diagnosed as AKI by their physician at discharge (Supplemental Table 2). The diagnosis rate in infants was extremely low (1%) compared with that in adolescents (11%). Among the patients with diagnosed AKI, the diagnosis rates by diagnostic code were 1%, 3%, and 9% for AKI stage 1, 2, and 3, respectively.
Risk Factors for AKI
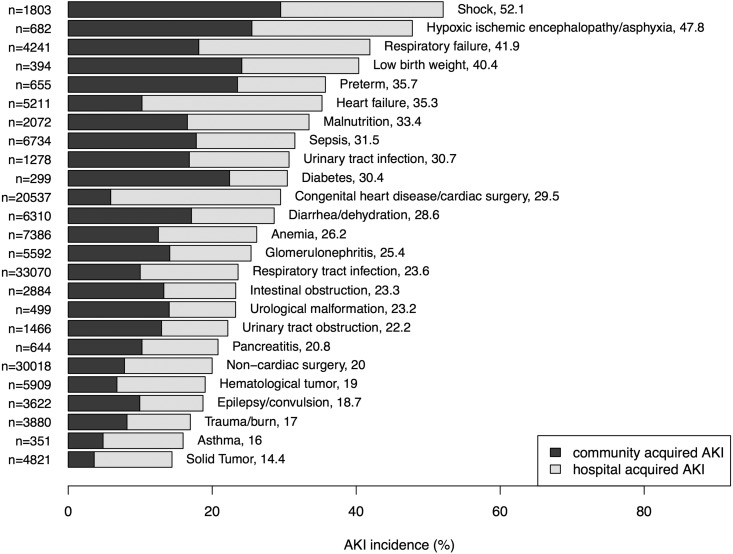
The cumulative incidence of community-acquired AKI and hospital-acquired AKI in various clinical settings is depicted in Figure 2. The clinical setting with the highest occurrence of community-acquired AKI was shock from all causes (30%), followed by hypoxic ischemic encephalopathy (26%) and low birth weight (24%). For hospital-acquired AKI, the top three settings were heart failure (25%), respiratory failure (24%), and congenital heart disease/cardiac surgery (24%).
Figure 2.
The incidences of AKI varied across different clinical settings. The numbers labeled after the clinical setting were incidence of total AKI in percentage.
We estimated the PAFs of the risk factors to assess their contribution to hospital-acquired AKI and community-acquired AKI in regression analyses. Overall, the top three risk factors, ranked in order of decreasing PAFs, were congenital heart disease/cardiac surgery, noncardiac surgery, and GN for hospital-acquired AKI (Table 2), and diarrhea/dehydration, sepsis, and GN for community-acquired AKI (Table 3). The profiles of risk factors for both community-acquired and hospital-acquired AKI differed substantially among age groups. The major risk factors for community-acquired AKI were diarrhea/dehydration and sepsis in infants, and GN and respiratory infection in adolescents. For hospital-acquired AKI, congenital heart disease/cardiac surgery was the dominant risk factor in infants, contributing to 27% of the AKI risk. Shock, GN, and respiratory failure constituted the top three risk factors for hospital-acquired AKI in adolescents.
Table 2.
Major risk factors of hospital acquired AKI by age groups
| Risk Factors | All Ages | 1 mo to 1 yr | 2–10 yr | 11–18 yr | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Freq (%) | HRa (95% CI) | PAF (%) | Freq (%) | HRa (95% CI) | PAF (%) | Freq (%) | HRa (95% CI) | PAF (%) | Freq (%) | HRa (95% CI) | PAF (%) | |
| Congenital heart disease/cardiac surgery | 20 | 1.96 (1.89 to 2.04) | 19 | 37 | 1.93 (1.84 to 2.03) | 27 | 16 | 2.10 (1.96 to 2.24) | 13 | 5 | 1.65 (1.31 to 2.08) | 3 |
| Noncardiac surgery | 29 | 1.14 (1.09 to 1.19) | 4 | 29 | 1.13 (1.07 to 1.20) | 3 | 29 | 1.12 (1.05 to 1.20) | 3 | 32 | 1.06 (0.91 to 1.22) | 2 |
| GN | 5 | 1.56 (1.44 to 1.69) | 2 | 0.3 | 1.21 (0.79 to 1.85) | 0.1 | 6 | 1.50 (1.36 to 1.66) | 3 | 10 | 1.30 (1.08 to 1.56) | 3 |
| Respiratory failure | 4 | 1.27 (1.19 to 1.36) | 2 | 7 | 1.06 (0.97 to 1.16) | 0.5 | 3 | 1.66 (1.48 to 1.86) | 3 | 1 | 2.32 (1.78 to 3.02) | 4 |
| Shock | 1 | 1.74 (1.59 to 1.92) | 2 | 1 | 1.60 (1.38 to 1.85) | 1 | 1 | 1.58 (1.37 to 1.82) | 1 | 1 | 2.55 (1.97 to 3.30) | 4 |
| Heart failure | 5 | 1.17 (1.10 to 1.24) | 1 | 9 | 1.23 (1.14 to 1.32) | 3 | 3 | 1.06 (0.95 to 1.19) | 0.3 | 2 | 1.21 (0.91 to 1.61) | 0.9 |
| Urinary tract obstruction | 1 | 1.37 (1.19 to 1.57) | 0.3 | 2 | 1.63 (1.37 to 1.95) | 0.5 | 1 | 1.20 (0.93 to 1.55) | 0.1 | 1 | 0.93 (0.53 to 1.63) | — |
| Diabetes | 0.2 | 1.62 (1.11 to 2.37) | 0.1 | — | — | — | 0.2 | 1.90 (1.05 to 3.46) | 0.1 | 0.8 | 1.86 (1.09 to 3.19) | 0.5 |
Freq, frequency; HR, hazard ratio; 95% CI, 95% confidence interval; PAF, population attributable fraction; —, did not estimate due to insufficient number of observations.
Adjusted by age, sex, baseline serum creatinine, comorbidities, and clinical procedures and stratified by center and the need for intensive care.
Table 3.
Major risk factors of community-acquired AKI by age groups
| Risk Factors | All Ages | 1 mo to 1 yr | 2–10 yr | 11–18 yr | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Freq (%) | ORa (95% CI) | PAF (%) | Freq (%) | ORa (95% CI) | PAF (%) | Freq (%) | ORa (95% CI) | PAF (%) | Freq (%) | ORa (95% CI) | PAF (%) | |
| Diarrhea/dehydration | 6 | 1.91 (1.76 to 2.08) | 6 | 12 | 2.05 (1.85 to 2.28) | 9 | 4 | 1.74 (1.50 to 2.01) | 3 | 2 | 1.79 (1.25 to 2.58) | 2 |
| Sepsis | 7 | 1.69 (1.56 to 1.84) | 6 | 9 | 1.71 (1.52 to 1.92) | 7 | 6 | 1.55 (1.36 to 1.77) | 4 | 4 | 2.20 (1.68 to 2.87) | 5 |
| GN | 6 | 2.34 (2.12 to 2.57) | 5 | 0.4 | 2.84 (1.80 to 4.46) | 0.6 | 7 | 1.68 (1.47 to 1.93) | 4 | 12 | 3.08 (2.60 to 3.65) | 20 |
| Respiratory infection | 33 | 1.14 (1.07 to 1.21) | 4 | 45 | 1.15 (1.05 to 1.26) | 5 | 30 | 1.03 (0.94 to 1.12) | 0.9 | 17 | 1.43 (1.21 to 1.70) | 9 |
| Shock | 2 | 2.81 (2.49 to 3.17) | 4 | 2 | 2.88 (2.37 to 3.50) | 4 | 2 | 2.75 (2.30 to 3.29) | 4 | 2 | 2.86 (2.04 to 3.99) | 4 |
| Respiratory failure | 4 | 1.55 (1.40 to 1.71) | 3 | 8 | 1.53 (1.34 to 1.75) | 4 | 3 | 1.67 (1.41 to 1.98) | 3 | 2 | 1.43 (0.97 to 2.09) | 1 |
| Intestinal obstruction | 3 | 2.00 (1.77 to 2.26) | 2 | 4 | 2.07 (1.75 to 2.46) | 3 | 3 | 2.10 (1.72 to 2.55) | 2 | 2 | 1.54 (0.99 to 2.41) | 0.8 |
| Heart failure | 5 | 1.36 (1.21 to 1.52) | 2 | 10 | 1.33 (1.16 to 1.53) | 2 | 3 | 1.44 (1.16 to 1.79) | 1 | 2 | 1.39 (0.88 to 2.20) | 0.7 |
| Trauma/burn | 4 | 1.59 (1.40 to 1.81) | 1 | 1 | 1.31 (0.95 to 1.81) | 0.3 | 5 | 1.56 (1.33 to 1.83) | 2 | 4 | 1.70 (1.22 to 2.38) | 2 |
| Urinary tract obstruction | 1 | 2.20 (1.84 to 2.63) | 1 | 2 | 1.63 (1.22 to 2.17) | 0.6 | 1 | 3.26 (2.51 to 4.23) | 2 | 1 | 2.33 (1.50 to 3.62) | 2 |
| Anemia | 7 | 1.13 (1.04 to 1.23) | 1 | 10 | 1.11 (0.98 to 1.26) | 1 | 6 | 1.14 (0.99 to 1.31) | 1 | 5 | 1.29 (1.00 to 1.65) | 2 |
| Malnutrition | 2 | 1.38 (1.21 to 1.58) | 1 | 4 | 1.71 (1.46 to 1.99) | 3 | 1 | 0.87 (0.64 to 1.18) | NA | 0.6 | 0.40 (0.17 to 0.92) | NA |
| Epilepsy | 4 | 1.35 (1.20 to 1.52) | 1 | 3 | 1.57 (1.31 to 1.89) | 2 | 4 | 1.21 (1.01 to 1.44) | 0.7 | 2 | 1.55 (1.04 to 2.30) | 0.9 |
| Hypoxic ischemic encephalopathy | 0.7 | 1.89 (1.55 to 2.31) | 0.9 | 1 | 1.83 (1.40 to 2.41) | 1 | 0.6 | 1.77 (1.29 to 2.43) | 0.9 | — | — | — |
| Urinary tract infection | 1 | 1.55 (1.31 to 1.83) | 0.8 | 1 | 1.31 (0.98 to 1.75) | 0.4 | 1 | 1.68 (1.31 to 2.16) | 1 | 1 | 1.83 (1.25 to 2.68) | 2 |
| Preterm | 0.7 | 1.58 (1.20 to 2.09) | 0.6 | 2 | 1.21 (0.88 to 1.65) | 0.5 | — | — | — | — | — | — |
| Diabetes | 0.3 | 4.33 (3.21 to 5.86) | 0.6 | — | — | — | 0.2 | 3.45 (2.09 to 5.69) | 0.4 | 0.9 | 5.90 (3.94 to 8.82) | 3 |
| Hematologic malignancy | 6 | 1.12 (1.00 to 1.26) | 0.5 | 0.5 | 1.21 (0.73 to 2.00) | 0.1 | 8 | 1.11 (0.96 to 1.28) | 0.7 | 9 | 1.13 (0.89 to 1.44) | 1 |
Freq, frequency; OR, odds ratio; 95% CI, 95% confidence interval; PAF, population attributable fraction; —, did not estimate due to insufficient number of observations.
Adjusted by age, sex, baseline serum creatinine, comorbidities, center, and the need for intensive care.
Use of nephrotoxic drugs was common in our study cohort and contributed to a large fraction of hospital-acquired AKI (Table 4). Use of nonsteroidal anti-inflammatory drugs (NSAIDs) and proton pump inhibitors (PPIs) (with an exposure rate of 23% and 27%, respectively) were associated with 63% (OR, 1.63; 95% confidence interval [95% CI], 1.44 to 1.85) and 52% (OR, 1.52; 95% CI, 1.34 to 1.73) higher odds for hospital-acquired AKI, contributing to 11% and 9% of hospital-acquired AKI, respectively. The list of the drugs analyzed is presented in the Supplemental Appendix.
Table 4.
Exposure to nephrotoxic drugs and the risk of hospital acquired AKI
| Drug | Frequency in All Patients (%) | Frequency in Patients with AKI (%) | ORa (95% CI) | PAF (%) |
|---|---|---|---|---|
| Nonsteroidal anti-inflammatory drugs | 23 | 32 | 1.63 (1.44 to 1.85) | 11 |
| Proton pump inhibitors | 27 | 30 | 1.52 (1.34 to 1.73) | 9 |
| Antimycotics | 4 | 8 | 2.09 (1.68 to 2.61) | 3 |
| Contrast media | 4 | 7 | 1.77 (1.41 to 2.22) | 3 |
| Aminoglycoside antibiotics | 4 | 5 | 1.63 (1.24 to 2.14) | 2 |
| Chemotherapeutic drugs | 3 | 4 | 1.32 (0.98 to 1.78) | 0.9 |
OR, odds ratio; 95% CI, 95% confidence interval; PAF, population attributable fraction.
Adjusted for the propensity score of drug use, time from admission, age, sex, center, baseline serum creatinine, comorbidities, clinical procedures, and need for intensive care.
In-Hospital Outcomes
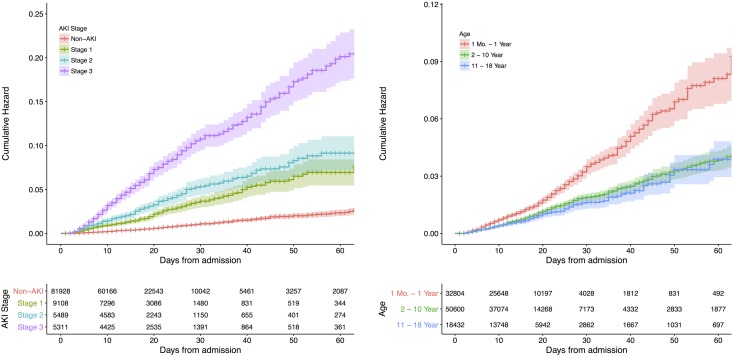
The incidence of in-hospital death was 0.5%, 2.3%, and 5.3% in children without AKI, with community-acquired AKI, and with hospital-acquired AKI, respectively (Table 1). The mortality was higher with greater severity of hospital-acquired AKI and lower with age (Figure 3). The HRs (95% CIs) of in-hospital death adjusted for age, sex, baseline serum creatinine, comorbidities, and surgical procedures were 3.2 (95% CI, 2.7 to 3.8), 4.8 (95% CI, 4.0 to 5.7), and 8.9 (95% CI, 7.6 to 10.4) for stage 1, stage 2, and stage 3 AKI, respectively (Table 5).
Figure 3.
The in-hospital mortality was higher with increasing number of days from admission and were higher in children with greater severity of AKI and those with younger age.
Table 5.
Major risk factors of in-hospital death
| Risk Factors | Survivors, N (%), n=100,544 | Nonsurvivors, N (%), n=1292 | Hazard Ratioa (95% CI) |
|---|---|---|---|
| AKI | |||
| Stage 1 | 8905 (9) | 203 (16) | 3.2 (2.9 to 3.7) |
| Stage 2 | 5292 (5) | 197 (15) | 4.6 (4.0 to 5.3) |
| Stage 3 | 4869 (5) | 442 (34) | 7.2 (6.3 to 8.3) |
| Respiratory failure | 3779 (4) | 462 (36) | 6.2 (5.4 to 7.0) |
| Shock | 1532 (2) | 271 (21) | 6.9 (6.0 to 7.9) |
| Hypoxic ischemic encephalopathy | 614 (0.6) | 68 (5) | 2.0 (1.6 to 2.6) |
| Sepsis | 6466 (6) | 268 (21) | 1.6 (1.4 to 1.9) |
| Respiratory infection | 32,432 (32) | 638 (49) | 1.3 (1.2 to 1.5) |
95% CI, 95% confidence interval.
Adjusted for age, sex, comorbidities, and clinical procedures.
AKI was associated with longer LOS and higher daily cost during hospitalization (Table 1). After adjusting for age, sex, comorbidities, and surgical procedures, community-acquired AKI and hospital-acquired AKI were associated with 10.9% and 16.1% longer LOS, and 8.8% and 18.1% higher daily cost, respectively, compared with those without AKI (P<0.001 for all).
Kidney Recovery
Among 7696 patients with hospital-acquired AKI with one or more serum creatinine tests after the onset of AKI, 4762 (61.9%) recovered kidney function before discharge. The median recovery time in patients with stage 3 AKI was 13.7 (95% CI, 12.3 to 15.0) days, compared with 7.0 (95% CI, 6.8 to 7.1) and 7.3 (95% CI, 7.0 to 8.0) days in patients with stage 1 and 2, respectively (Supplemental Figure 2).
Discussion
This study represents the largest and the most extensive epidemiologic description of pediatric AKI in developing countries, encompassing a wide disease spectrum. We estimated an occurrence of 7% for community-acquired AKI and an accumulative incidence of 13% for hospital-acquired AKI during hospitalization, using the KDIGO creatinine criteria. Up to 96% of these AKI events were not diagnosed on the discharge records, suggesting substantial underdiagnosis. We calculated the contributions of potential risk factors to pediatric AKI and found that the majority of risk factors were clinically preventable. We also found that exposure to nephrotoxic drugs was common in hospitalized children and contributed to a large proportion of AKI cases in the study cohort.
Previously reported incidences of pediatric AKI vary widely from 0.3% to 82% (11,20,31,40,41). This variation may result from differences in case mix, confounders, disease severity, and diagnostic criteria of AKI. The cumulative incidence of AKI in our study population (20%) was much higher than the range of 0.39%–1.4% reported in several studies using ICD coding for pediatric AKI diagnosis (19,42), but lower than that (40.3%) reported by a single-center study (40). In our study, 35% of children developed AKI during their intensive care unit stay, the incidence of AKI in the intensive care population was also higher than those (11.8%–26.9%) reported in recent large-scale studies of critically ill children, which use the same diagnostic criteria (1,22,41). We found that the cumulative incidence of AKI in infants (28%) doubled that of adolescents (12%). The greatest incidence of AKI is among infants aged 1 month to 1 year. This trend is consistent with previous reports using the serum creatinine criteria (26,43,44), but is in contrast with the study using the coding method (43).
Our study results provide informative comparisons with those from the adults in the EACH study (36). The incidence of AKI (12%) was lower in the adult study than the corresponding rates in this study. Although both studies show a higher risk of in-hospital mortality with increasing KDIGO stages, this study shows a lower overall mortality (4%) among children with AKI than the adults in the EACH study (9%). These data suggest a possibility that children are more likely to survive AKI than adults. The multiple confounders that exist in adults may increase susceptibility to AKI-related mortality and morbidity. The mortality rate in our population is substantially lower than that reported in a United States national cohort (4% versus 10%), partly due to fewer patients in the intensive care unit (15% versus 23%) (42). Interestingly, the kidney recovery rate of AKI in children (61%) is higher than that in adults (40%) in the EACH study, using a strict definition of kidney recovery.
Studies on community-acquired AKI in children remain scarce. We identified a total of 7220 patients with community-acquired AKI in our cohort. The profiles of risk factors differed markedly between community-acquired and hospital-acquired AKI and varied with age. The top risk factor contributing to community-acquired AKI in infants was diarrhea/dehydration, followed by sepsis. The top risk factor switched to GN after 2 years of age, whereas infectious diseases remained a major player. Given that diarrhea/dehydration and infection are usually reversible under appropriate treatment, efforts on the prevention of AKI should focus on primary health care. Community physicians need to be able to recognize the risk factors of AKI, to properly manage infection and dehydration, and to timely refer patients requiring critical care to tertiary hospitals.
Exposure to nephrotoxic drugs has been recognized as a risk factor for pediatric AKI (26). In our cohort, more than 30% of AKI was attributable to exposure of nephrotoxic drugs. Notably, exposure of NSAIDs and PPIs were the most important risk factors, contributing to 11% and 9% of risk for hospital-acquired AKI, respectively. In this study, 27% of hospitalized children received PPIs and 23% were treated with NSAIDs within the week before the 7-day serum creatinine test window. The high exposure to NSAIDs might be related to the fact that many physicians in China used to prescribe NSAIDs for management of febrile illness in children. Similarly, PPIs are widely used for acid inhibition before surgery. Although the nephrotoxicity of these drugs has been reported in adults (45–48), we demonstrate for the first time that exposure to PPIs is associated with higher risk of pediatric AKI. Avoiding inappropriate use of these drugs would be an important strategy for preventing hospital-acquired AKI in children.
Among 19,908 children with AKI identified by screening the serum creatinine data, only 4% of the patients were diagnosed as AKI on discharge records, suggesting that the vast majority of AKI events were not recognized by clinicians. Under-reporting of AKI, especially mild AKI, may also contribute to the low diagnosis rate. The physician-diagnosis rate of AKI was particularly lower among children in infancy (1%) and childhood (4%) compared with those in adolescence (11%), as well as those previously reported in the Chinese adults (26%) (49). The low awareness of pediatric AKI in medical practice is also supported by the observation that a large proportion of inpatients received insufficient creatinine testing during hospitalization. The underestimation of AKI burden may lead to a lack of attention for this disease from the public and the government.
Our study has a number of strengths. It involves a large, multicenter cohort of children, which enabled a robust evaluation of association between exposure and outcomes. Second, the availability of patient-level data with time stamps permits a detailed examination of risk profiles and adjustment for possible confounders. Third, we distinguished community-acquired and hospital-acquired AKI in the risk factor analyses, providing evidence for preventing AKI at the primary care level.
Our study has several limitations. First, the majority of the hospitalized pediatric patients did not have enough serum creatinine measurements to be screened for AKI status. However, we used a probability sampling model to estimate the AKI incidence in the whole cohort, assuming that patients had equal chances of receiving creatinine test if they had identical comorbidities, baseline creatinine level, length of hospitalization stay, need for intensive care, and study center. Second, the study centers were not selected by random sampling and may subject to selection bias. Our study sites were regional central hospitals covering 15 provinces cross China, including ten of the top 15 hospitals in pediatrics. The incidence and risk profiles of hospital-acquired AKI in lower-level hospitals may differ. Third, the majority of the hospitalized children did not have serum creatinine data before hospitalization. Using in-hospital serum creatinine for the estimation of the baseline may lead to underestimation of AKI incidence (if the serum creatinine was already starting to be elevated at admission). Fourth, we were not able to use urine output for AKI identification because these data were not available in our cohort. This may result in underestimation of AKI incidence.
In conclusion, pediatric AKI has become a public health problem and represents a big economic burden in China. The disease burden of pediatric AKI is significantly underestimated. Raising the awareness of pediatric AKI and its risk factors among physicians, especially primary care providers, will improve health care in children worldwide.
Disclosures
None.
Supplementary Material
Acknowledgments
F.F.H. and X.X. contributed to the study design and results interpretation. F.F.H. took the lead in drafting the manuscript and received major funding for the study. X.X. and S.N. obtained and analyzed the data. S.N., P.H., and W.H. prepared and cleaned the data. A.Z., J.M., H.-P.L., H. Xia, H. Xu, Z.L., S.F., W.Z., X.L., Y.Y., Y. Tao, Y.F., C.C., M.W., Y.Z., J.-H.F., Q.L., S.G., J.C., Y.H., S.T., C.H., B.-C.L., and Y. Tang contributed to the data collection. All authors contributed to interpretation of data, provided critical revisions to the manuscript, and approved the final draft.
This work was supported by the National Key Technology Support Program of China (grant 2015BAI12B07 to F.F.H), the National Natural Science Foundation of China (Key Program) (grant 81430016 to F.F.H), the National Natural Science Foundation of China (grant 81770683 to X.X.), the Major Scientific and Technological Planning Project of Guangzhou (grant 201504010027 to F.F.H), and the Major International (Regional) Joint Research Project (grant 81620108003 to F.F.H).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.00800118/-/DCSupplemental.
References
- 1.Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL; AWARE Investigators : Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med 376: 11–20, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellomo R, Kellum JA, Ronco C: Acute kidney injury. Lancet 380: 756–766, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C; Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators : Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA 294: 813–818, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Susantitaphong P, Cruz DN, Cerda J, Abulfaraj M, Alqahtani F, Koulouridis I, Jaber BL; Acute Kidney Injury Advisory Group of the American Society of Nephrology : World incidence of AKI: A meta-analysis. Clin J Am Soc Nephrol 8: 1482–1493, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Volpon LC, Sugo EK, Consulin JC, Tavares TL, Aragon DC, Carlotti AP: Epidemiology and outcome of acute kidney injury according to pediatric risk, injury, failure, loss, end-stage renal disease and kidney disease: Improving global outcomes criteria in critically ill children-a prospective study. Pediatr Crit Care Med 17: e229–e238, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Mammen C, Al Abbas A, Skippen P, Nadel H, Levine D, Collet JP, Matsell DG: Long-term risk of CKD in children surviving episodes of acute kidney injury in the intensive care unit: A prospective cohort study. Am J Kidney Dis 59: 523–530, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW: Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 16: 3365–3370, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Sinha R, Nandi M, Tullus K, Marks SD, Taraphder A: Ten-year follow-up of children after acute renal failure from a developing country. Nephrol Dial Transplant 24: 829–833, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Askenazi DJ, Feig DI, Graham NM, Hui-Stickle S, Goldstein SL: 3-5 year longitudinal follow-up of pediatric patients after acute renal failure. Kidney Int 69: 184–189, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Ingelfinger JR, Kalantar-Zadeh K, Schaefer F; World Kidney Day Steering Committee : Averting the legacy of kidney disease: Focus on childhood. Nephrol Dial Transplant 31: 327–331, 2016 [DOI] [PubMed] [Google Scholar]
- 11.Lameire N, Van Biesen W, Vanholder R: Epidemiology of acute kidney injury in children worldwide, including developing countries. Pediatr Nephrol 32: 1301–1314, 2017 [DOI] [PubMed] [Google Scholar]
- 12.Lameire NH, Bagga A, Cruz D, De Maeseneer J, Endre Z, Kellum JA, Liu KD, Mehta RL, Pannu N, Van Biesen W, Vanholder R: Acute kidney injury: An increasing global concern. Lancet 382: 170–179, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Lewington AJ, Cerdá J, Mehta RL: Raising awareness of acute kidney injury: A global perspective of a silent killer. Kidney Int 84: 457–467, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehta RL, Cerdá J, Burdmann EA, Tonelli M, García-García G, Jha V, Susantitaphong P, Rocco M, Vanholder R, Sever MS, Cruz D, Jaber B, Lameire NH, Lombardi R, Lewington A, Feehally J, Finkelstein F, Levin N, Pannu N, Thomas B, Aronoff-Spencer E, Remuzzi G: International Society of Nephrology’s 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): A human rights case for nephrology. Lancet 385: 2616–2643, 2015 [DOI] [PubMed] [Google Scholar]
- 15.Remuzzi G, Horton R: Acute renal failure: An unacceptable death sentence globally. Lancet 382: 2041–2042, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL: Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int 71: 1028–1035, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Schneider J, Khemani R, Grushkin C, Bart R: Serum creatinine as stratified in the RIFLE score for acute kidney injury is associated with mortality and length of stay for children in the pediatric intensive care unit. Crit Care Med 38: 933–939, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Vachvanichsanong P, Dissaneewate P, Lim A, McNeil E: Childhood acute renal failure: 22-year experience in a university hospital in southern Thailand. Pediatrics 118: e786–e791, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Chang JW, Jeng MJ, Yang LY, Chen TJ, Chiang SC, Soong WJ, Wu KG, Lee YS, Wang HH, Yang CF, Tsai HL: The epidemiology and prognostic factors of mortality in critically ill children with acute kidney injury in Taiwan. Kidney Int 87: 632–639, 2015 [DOI] [PubMed] [Google Scholar]
- 20.Bailey D, Phan V, Litalien C, Ducruet T, Mérouani A, Lacroix J, Gauvin F: Risk factors of acute renal failure in critically ill children: A prospective descriptive epidemiological study. Pediatr Crit Care Med 8: 29–35, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Hui-Stickle S, Brewer ED, Goldstein SL: Pediatric ARF epidemiology at a tertiary care center from 1999 to 2001. Am J Kidney Dis 45: 96–101, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Selewski DT, Cornell TT, Heung M, Troost JP, Ehrmann BJ, Lombel RM, Blatt NB, Luckritz K, Hieber S, Gajarski R, Kershaw DB, Shanley TP, Gipson DS: Validation of the KDIGO acute kidney injury criteria in a pediatric critical care population. Intensive Care Med 40: 1481–1488, 2014 [DOI] [PubMed] [Google Scholar]
- 23.Ball EF, Kara T: Epidemiology and outcome of acute kidney injury in New Zealand children. J Paediatr Child Health 44: 642–646, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Zappitelli M, Greenberg JH, Coca SG, Krawczeski CD, Li S, Thiessen-Philbrook HR, Bennett MR, Devarajan P, Parikh CR; Translational Research Investigating Biomarker Endpoints in Acute Kidney Injury (TRIBE-AKI) Consortium : Association of definition of acute kidney injury by cystatin C rise with biomarkers and clinical outcomes in children undergoing cardiac surgery. JAMA Pediatr 169: 583–591, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rheault MN, Zhang L, Selewski DT, Kallash M, Tran CL, Seamon M, Katsoufis C, Ashoor I, Hernandez J, Supe-Markovina K, D’Alessandri-Silva C, DeJesus-Gonzalez N, Vasylyeva TL, Formeck C, Woll C, Gbadegesin R, Geier P, Devarajan P, Carpenter SL, Kerlin BA, Smoyer WE; Midwest Pediatric Nephrology Consortium : AKI in children hospitalized with nephrotic syndrome. Clin J Am Soc Nephrol 10: 2110–2118, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moffett BS, Goldstein SL: Acute kidney injury and increasing nephrotoxic-medication exposure in noncritically-ill children. Clin J Am Soc Nephrol 6: 856–863, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou YM, Yin XL, Huang ZB, He YH, Qiu LR, Zhou JH: Risk factors and prognostic factors of acute kidney injury in children: A retrospective study between 2003 and 2013. J Huazhong Univ Sci Technolog Med Sci 35: 785–792, 2015 [DOI] [PubMed] [Google Scholar]
- 28.Hui WF, Chan WK, Miu TY: Acute kidney injury in the paediatric intensive care unit: Identification by modified RIFLE criteria. Hong Kong Med J 19: 13–19, 2013 [PubMed] [Google Scholar]
- 29.Zheng J, Xiao Y, Chong M, Chen Y, Yao Y, Jin M, Liu Y, Han L: The effect of cardiopulmonary bypass duration on renal injury after congenital heart surgery in infants and young children. Adv Clin Exp Med 22: 693–698, 2013 [PubMed] [Google Scholar]
- 30.Cao Y, Yi ZW, Zhang H, Dang XQ, Wu XC, Huang AW: Etiology and outcomes of acute kidney injury in Chinese children: A prospective multicentre investigation. BMC Urol 13: 41–48, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGregor TL, Jones DP, Wang L, Danciu I, Bridges BC, Fleming GM, Shirey-Rice J, Chen L, Byrne DW, Van Driest SL: Acute kidney injury incidence in noncritically ill hospitalized children, adolescents, and young adults: A retrospective observational study. Am J Kidney Dis 67: 384–390, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liangos O, Wald R, O’Bell JW, Price L, Pereira BJ, Jaber BL: Epidemiology and outcomes of acute renal failure in hospitalized patients: A national survey. Clin J Am Soc Nephrol 1: 43–51, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Waikar SS, Wald R, Chertow GM, Curhan GC, Winkelmayer WC, Liangos O, Sosa MA, Jaber BL: Validity of international classification of diseases, ninth revision, clinical modification codes for acute renal failure. J Am Soc Nephrol 17: 1688–1694, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Lafrance JP, Miller DR: Defining acute kidney injury in database studies: The effects of varying the baseline kidney function assessment period and considering CKD status. Am J Kidney Dis 56: 651–660, 2010 [DOI] [PubMed] [Google Scholar]
- 35.KDIGO AKI Work Group : KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2: 1–138, 2012 [Google Scholar]
- 36.Xu X, Nie S, Liu Z, Chen C, Xu G, Zha Y, Qian J, Liu B, Han S, Xu A, Xu X, Hou FF: Epidemiology and clinical correlates of AKI in Chinese hospitalized adults. Clin J Am Soc Nephrol 10: 1510–1518, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.World Health Organization Collaborating Centre for Drug Statistics Methodology: Anatomical Therapeutic Chemical (ATC) Classification Index with Defined Daily Doses (DDDs), 2017. Available at: http://www.whocc.no/atcddd/. Accessed February 1, 2017
- 38.Lumley T: Analysis of complex survey samples. J Stat Softw 9: 1–19, 2004 [Google Scholar]
- 39.Bruzzi P, Green SB, Byar DP, Brinton LA, Schairer C: Estimating the population attributable risk for multiple risk factors using case-control data. Am J Epidemiol 122: 904–914, 1985 [DOI] [PubMed] [Google Scholar]
- 40.Sutherland SM, Byrnes JJ, Kothari M, Longhurst CA, Dutta S, Garcia P, Goldstein SL: AKI in hospitalized children: Comparing the pRIFLE, AKIN, and KDIGO definitions. Clin J Am Soc Nephrol 10: 554–561, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanchez-Pinto LN, Goldstein SL, Schneider JB, Khemani RG: Association between progression and improvement of acute kidneyinjury and mortality in critically ill children. Pediatr Crit Care Med 16: 703–710, 2015 [DOI] [PubMed] [Google Scholar]
- 42.Sutherland SM, Ji J, Sheikhi FH, Widen E, Tian L, Alexander SR, Ling XB: AKI in hospitalized children: Epidemiology and clinical associations in a national cohort. Clin J Am Soc Nephrol 8: 1661–1669, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zappitelli M, Bernier PL, Saczkowski RS, Tchervenkov CI, Gottesman R, Dancea A, Hyder A, Alkandari O: A small post-operative rise in serum creatinine predicts acute kidney injury in children undergoing cardiac surgery. Kidney Int 76: 885–892, 2009 [DOI] [PubMed] [Google Scholar]
- 44.Chiravuri SD, Riegger LQ, Christensen R, Butler RR, Malviya S, Tait AR, Voepel-Lewis T: Factors associated with acute kidney injury or failure in children undergoing cardiopulmonary bypass: A case-controlled study. Paediatr Anaesth 21: 880–886, 2011 [DOI] [PubMed] [Google Scholar]
- 45.Nochaiwong S, Ruengorn C, Awiphan R, Koyratkoson K, Chaisai C, Noppakun K, Chongruksut W, Thavorn K: The association between proton pump inhibitor use and the risk of adverse kidney outcomes: A systematic review and meta-analysis. Nephrol Dial Transplant 33: 331–342 2018 [DOI] [PubMed] [Google Scholar]
- 46.Lazarus B, Chen Y, Wilson FP, Sang Y, Chang AR, Coresh J, Grams ME: Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med 176: 238–246, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xie Y, Bowe B, Li T, Xian H, Balasubramanian S, Al-Aly Z: Proton pump inhibitors and risk of incident CKD and progression to ESRD. J Am Soc Nephrol 27: 3153–3163, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dreischulte T, Morales DR, Bell S, Guthrie B: Combined use of nonsteroidal anti-inflammatory drugs with diuretics and/or renin-angiotensin system inhibitors in the community increases the risk of acute kidney injury. Kidney Int 88: 396–403, 2015 [DOI] [PubMed] [Google Scholar]
- 49.Yang L, Xing G, Wang L, Wu Y, Li S, Xu G, He Q, Chen J, Chen M, Liu X, Zhu Z, Yang L, Lian X, Ding F, Li Y, Wang H, Wang J, Wang R, Mei C, Xu J, Li R, Cao J, Zhang L, Wang Y, Xu J, Bao B, Liu B, Chen H, Li S, Zha Y, Luo Q, Chen D, Shen Y, Liao Y, Zhang Z, Wang X, Zhang K, Liu L, Mao P, Guo C, Li J, Wang Z, Bai S, Shi S, Wang Y, Wang J, Liu Z, Wang F, Huang D, Wang S, Ge S, Shen Q, Zhang P, Wu L, Pan M, Zou X, Zhu P, Zhao J, Zhou M, Yang L, Hu W, Wang J, Liu B, Zhang T, Han J, Wen T, Zhao M, Wang H; ISN AKF 0by25 China Consortiums : Acute kidney injury in China: A cross-sectional survey. Lancet 386: 1465–1471, 2015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.