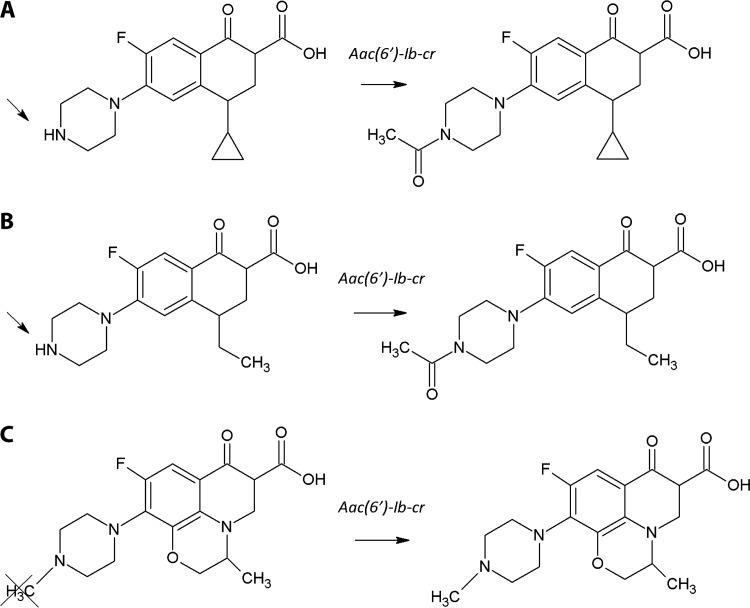
FIG 2.
Acetylation reaction in fluoroquinolones. (Republished from reference 73 with permission of the British Society for Antimicrobial Chemotherapy.) Shown are effects of the aac(6′)-Ib-cr gene on the chemical structures of ciprofloxacin (A), norfloxacin (B), and levofloxacin (C). Acetylation occurs at the amino nitrogen on the piperazinyl substituent of fluoroquinolones, a structural feature shared by ciprofloxacin and norfloxacin but not levofloxacin. Acetylation yields an increase of 43 Da in the mass of the antibiotic, which can be measured by MS.