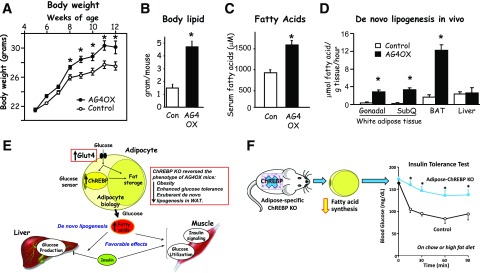
Figure 6.
Role of GLUT4 in the regulation of adiposity and lipid metabolism. Adipose-specific overexpression of GLUT4 (AG4OX) results in increased body weight (A), adiposity (B), serum free fatty acids (C), and de novo lipogenesis in white and brown adipose depots but not in liver (D). Panels A and B reprinted from Shepherd et al. (3). Panel C adapted from Table 2 in Carvalho et al. (4). In panel D, some data are adapted from Herman et al. (28). E: Increasing glucose transport in adipocytes enhances systemic insulin sensitivity by channeling glucose into fatty acid synthesis. When GLUT4 protein levels increase, ChREBP senses the increased glucose transport, leading to alterations in adipocyte biology, including driving more glucose into fatty acid synthesis (also known as de novo lipogenesis). That process has favorable effects on insulin action to suppress hepatic glucose production and on insulin signaling and glucose utilization in muscle. Knocking out (KO) ChREBP reverses the increased fatty acid synthesis and the beneficial effects of increased GLUT4 expression in adipocytes (see box insert). M.A. Herman contributed to the original design of panel E. F: Adipose-specific KO of ChREBP in otherwise normal mice reduces fatty acid synthesis and causes systemic insulin resistance. Data in panel F was reprinted from Vijayakumar et al. (29). For experimental details for panels A and B, see Shepherd et al. (3); for panel C, see Carvalho et al. (4); for panel D, see Herman et al. (28); and for panel F, see Vijayakumar et al. (29). BAT, brown adipose tissue; Con, control; SubQ, subcutaneous adipose tissue. *Panels A–D and F: P < 0.05 vs. control.