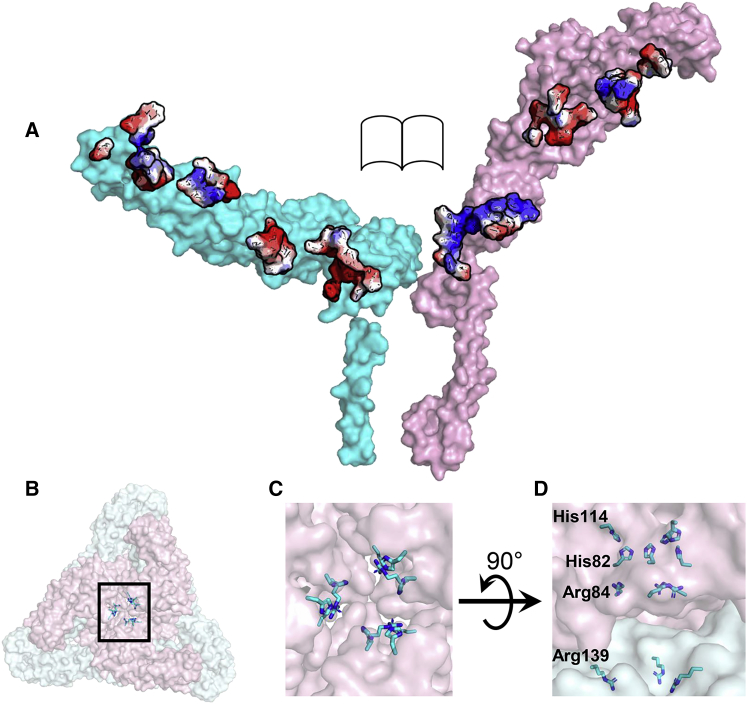
Figure 5.
Charged Residues at the E1-E2 Interface
(A) Open book view of E1 (cyan) and E2 (pink) proteins showing complementary charged residues at the E1-E2 dimer interface. Acidic and basic residues are colored red and blue, respectively.
(B–D) Basic residues in the E2-E2 interface formed by three symmetry-related E2 ectodomains (pink), along the spike 3-fold axis.
(B) Trimeric spike along 3-fold axis. The black box encloses the basic residues in the E2-E2 interface.
(C) Magnified view of the black box from (B).
(D) The four basic residues along the E2-E2 interface from three symmetry-related E2 molecules.
See also Figures S4 and S7.