Abstract
The objective of this study was to evaluate task performance and handling errors with soft mist inhalers (SMIs) or pressurized metered-dose inhalers (pMDIs) among patients with chronic obstructive pulmonary disease (COPD) experienced with, but not recently trained in, using these devices. This exploratory, noninterventional, simulated-use study (D5970R00004) assessed handling/usability of SMIs and pMDIs in inhaler-experienced patients with COPD (40–78 years; diagnosis ≥6 months). Patients received a device and instruction-for-use leaflet but no training and were recorded while performing tasks required for checking the device, priming, and dosing. Errors that could substantially affect the lung-delivered dose were considered critical. Sixteen of 61 patients (52% male) had used SMIs and 55 had used pMDIs. Thirty-one patients received an SMI and 30 a pMDI. Overall, 79% made ≥5 performance errors (SMI 94%; pMDI 63%) and 49% made ≥5 critical errors (SMI 68%; pMDI 30%). All patients made ≥1 error; three (all pMDI) made no critical errors. Regardless of the device used and previous inhaler experience, patient-centered training, education, and continuous retraining on correct inhaler use should be key aspects of routine patient care in COPD.
Keywords: Chronic obstructive pulmonary disease, bronchodilator agents, inhalation, metered dose inhalers, soft mist inhalers, patient compliance
Introduction
Chronic obstructive pulmonary disease (COPD), characterized by persistent respiratory symptoms and airflow limitation, is a leading cause of morbidity and mortality worldwide.1
Inhalers are key in delivery of treatment for COPD1; however, handling errors potentially affecting drug delivery to the lungs are common with all device types2–6 and are associated with poor treatment outcomes, including increased risks of severe COPD exacerbations, health care resource use, and poor disease control.2,7 Consequently, poor inhalation technique is thought to contribute considerably to COPD management costs.8 Factors associated with poor inhaler technique include older age, lower education level, gender, and lack of training from health care professionals.7,9–11
Previous studies have investigated device handling and user errors for dry powder inhalers (DPIs) and pressurized metered dose inhalers (pMDIs).4–6,12 Use of these devices requires different inhalation techniques. DPIs are breath-actuated and typically require forceful inhalation, while pMDIs require a deep and slow inhalation that is coordinated with device actuation.13
Handling errors with another device type, the soft mist inhaler (SMI), are less well characterized. Similar to pMDIs, SMIs also require coordination of inhalation with actuation.14 Most studies comparing SMIs and pMDIs focus on treatment efficacy and/or lung deposition, and patients are trained in correct device use as part of the study.15–18 Few studies have focused on handling errors, specifically with SMIs.
Our study investigated the device handling and usability of a pMDI and an SMI by inhaler-experienced patients with COPD who were not specifically trained on the use of these devices as part of this study, in order to characterize the frequency and type of error that such patients make when using these devices.
Methods
Study design
This study was an exploratory noninterventional, parallel-group, simulated-use study of inhaler-experienced participants with COPD, in a setting mimicking real-world use (Study ID: D5970R00004). The main study objective was to assess device handling and usability of a pMDI and an SMI by closely observing participants’ task performance and to investigate the causes of any observed errors, “close calls,” or difficulties.
Two devices were investigated: a pMDI and an SMI. Bevespi Aerosphere® was used to represent a pMDI with an integrated dose counter and was presented with a reprinted label excluding the drug and brand names and expiry date (devices with labels including an expiry date were used for tasks where date recognition was assessed). Instructions for use were also reprinted to remove drug and brand information.
The SMI (Respimat®) was presented as packaged, with brand and drug information visible and the instructions for use (two inhalations/dose). Participants were assigned to one of the two device groups at the close of recruitment in an approximate balance of age, gender, and previous device-use experience between user groups. Participants were asked to engage in a series of tasks aimed at simulating device priming and use steps.
The study was performed in accordance with ethical principles consistent with the Declaration of Helsinki, International Conference on Harmonization Good Clinical Practice, guidelines for Good Pharmacoepidemiology Practices and the applicable legislation on noninterventional studies. The study was conducted as market research guided by the British Healthcare Business Intelligence Association’s legal and ethical guidelines and, as such, did not require ethics approval.19
Participants
Participants were 40–80 years of age, with a ≥6-month history of COPD and were currently prescribed a pMDI or an SMI. Additionally, they were physically and cognitively able to read written English instructions without caregiver aid (score of ≥4 on the Rapid Estimate of Adult Literacy in Medicine–Short Form test at screening).
Exclusion criteria were noncorrectable low vision or blindness, severe dexterity loss, and requirement of assistance with day-to-day activities. Participants previously involved in inhaled therapy market research within the last 3 months or who worked or had close family working in the pharmaceutical or market research industries were also excluded. Patients were excluded if it was felt that participation may constitute a safety issue due to COPD or comorbid health conditions, or if the participant had known hypersensitivity to tiotropium, ipratropium, or olodaterol.
Device handling assessment
First, participants were presented with a fully functional inhaler and asked to “prepare for first use” and instructed to direct the plume into the fume hood during priming. Second, the participants were presented with an empty inhaler of the same type as before but without any active pharmaceutical ingredient (an empty canister (pMDI) and no canister (SMI)) and asked to simulate performing two inhalations. Third, participants were questioned on their understanding of the need to reprime the device. Finally, participants were presented with five devices and asked to sort them into two groups: devices they could continue to use and devices they could not. Of these five devices, one was full but had expired; one was half full; one was nearing the reorder dose and was expired; one had exceeded the reorder dose; and one was empty. Each participant received only one type of inhaler (either a pMDI or an SMI) during the study.
In the simulated-use study, participants were provided with the device and instructions for use but were not specifically directed to refer to them. The session was led by an experienced moderator, who spoke with one participant at a time following a discussion guide, while a trained observer watched from behind mirrored glass. Tasks were conducted with minimal input from the moderator, and participants were observed until signaling completion. If the participant displayed potentially harmful behavior, particularly if the active device was activated into the air, near the face, or placed in or near the mouth, the moderator intervened. Each participant’s activities were recorded by the moderator and the observer via an observation checklist and by three video cameras. Checklists were constructed using the task steps detailed in the Bevespi Aerosphere and Respimat prescribing information.20,21 To focus on the participant’s response, the moderator completed the checklist by omission, recording use errors, close calls, and task difficulties only. The participant was shown the recording of their completion of the tasks after task completion, and if the moderator noted any deviation from acceptable task performance (detailed in the Online Supplementary Material), they discussed with the participant what may have caused that behavior.
Task error classification
Task errors were categorized into two types: critical errors and significant errors. Errors were classified as critical where task failure may substantially affect the dose delivered to the lung, similar to a previous study.2 Significant errors were task failures that could be considered critical based on the general understanding of the device’s operational characteristics and the authors’ expert consensus, but where no evidence was currently available.
Task performance
Task performance was evaluated in three categories based on the US Food and Drug Administration (FDA) guidance on Applying Human Factors and Usability Engineering to Medical Devices: task failure, task difficulty, or correct use.22 A full description of task failures and task difficulties for each device is provided in the Online Supplementary Material.
Statistical analyses
Reporting of study results was primarily qualitative. All use errors and incorrect responses were reported and their nature and root causes explored. Quantitative analysis of the results was summarized using percentage and descriptive measures where possible. A sample size of 60 participants was planned, which was above the minimum sample size recommendation of 15 patients per distinct user group for validation testing in the FDA guidance on Applying Human Factors and Usability Engineering to Medical Devices.22
Results
Study population
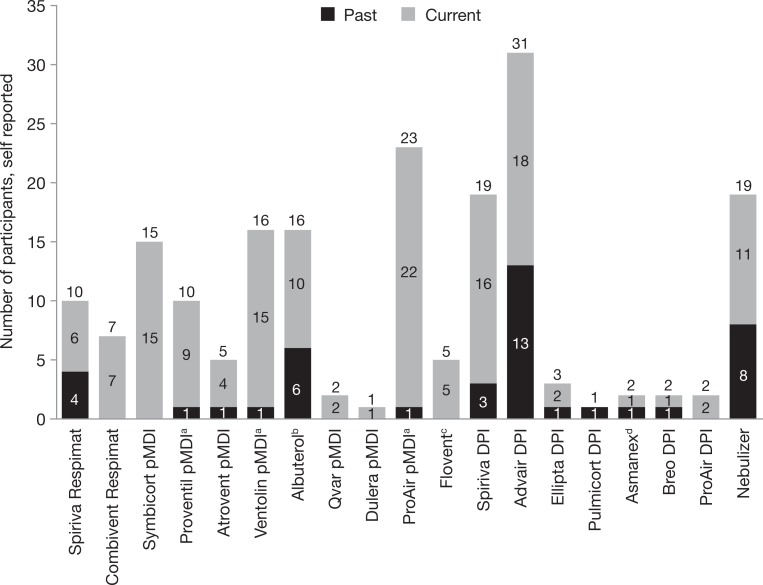
Sixty-one participants (40–78 years of age) took part at two study centers in Atlanta (GA, USA) and Boston (MA, USA) in November 2016. Of these, 30 used the pMDI and 31 used the SMI (Table 1). Participants had current and past experience with various inhaler types, including pMDIs, SMIs, and DPIs as well as nebulizers (Figure 1; summarized in Table 2). There was a difference in COPD severity between groups; more pMDI participants had moderate–severe COPD (pMDI 37%; SMI 10%). At the time of the study, 53 participants were current users of a pMDI and 13 were current users of an SMI. Additionally, two participants had previously used a pMDI and three had previously used an SMI.
Table 1.
Participant characteristics.
| Device group | All (N = 61) | pMDI (N = 30) | SMI (N = 31) |
|---|---|---|---|
| Male, n (%) | 32 (52) | 18 (60) | 14 (45) |
| Age at study (years) | |||
| Male, mean (SD) | 59.8 (10.2) | 59.8 (9.9) | 59.8 (10.9) |
| Female, mean (SD) | 60.7 (7.8) | 63.8 (8.4) | 58.5 (6.8) |
| Range | 40–78 | 41–78 | 40–76 |
| Time since diagnosis (years) | |||
| Male, mean (min) | 8.5 (0.8) | 10.2 (1.0) | 6.4 (0.8) |
| Female, mean (min) | 8.0 (1.0) | 8.8 (1.0) | 7.4 (2.0) |
| COPD severity, self-reported level, n (%) | |||
| Moderate–severe | 14 (23) | 11 (37) | 3 (10) |
| Moderate | 30 (49) | 13 (43) | 17 (55) |
| Mild–moderate | 17 (28) | 6 (20) | 11 (35) |
| REALM-SF scorea (1 worst, 7 max) | |||
| Modea score | 7 | 7 | 7 |
| Minimum score [Participant #] | 4 [P35] | 4 [P35] | 5 [P39] |
COPD: chronic obstructive pulmonary disease; pMDI: pressurized metered dose inhaler; REALM-SF: Rapid Estimate of Adult Literacy in Medicine–Short Form; SD: standard deviation; SMI: soft mist inhaler.
a The mode is the score that was most frequently reported.
Figure 1.
Self-reported device use in study participants. N = 61. Numbers above the bars are the total number of past and present users of each device. aReported as brand name rather than albuterol pMDI. bTen participants had used albuterol pMDI, and for the others, the device type was not specified. cTwo participants had used Flovent pMDI, and for the others, the device type was not specified. dOne participant used Asmanex DPI, and the other participant did not specify the device type. DPI: dry powder inhaler; pMDI: pressurized metered dose inhaler.
Table 2.
Experience of device use.
| All (N = 61) | pMDI (N = 30) | SMI (N = 31) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Current user | Past user | All | Current user | Past user | All | Current user | Past user | All | |
| Number of patients using each device | |||||||||
| pMDI | 53 | 2 | 55 | 26 | 1 | 27 | 27 | 1 | 28 |
| Respimat SMI | 13 | 3 | 16 | 5 | 3 | 8 | 8 | 0 | 8 |
| Capsule inhaler (DPI) | 16 | 3 | 19 | 8 | 3 | 11 | 8 | 0 | 8 |
| Multidose DPI | 23 | 13 | 36 | 14 | 9 | 23 | 9 | 4 | 13 |
| Number of devices used by each patient | |||||||||
| Mean | 2.4 | 0.7 | 3.1 | 2.5 | 0.9 | 3.5 | 2.3 | 0.4 | 2.7 |
| Modea (range) | 2 (1–5) | 0 (0–3) | 3 (1–5) | 2 (1–5) | 0 (0–3) | 3 (1–5) | 2 (1–4) | 0 (0–2) | 3 (1–5) |
DPI: dry powder inhaler; pMDI pressurized metered dose inhaler; SMI soft mist inhaler.
a The mode is the most commonly reported number of devices a participant uses or has used.
Use of instructions
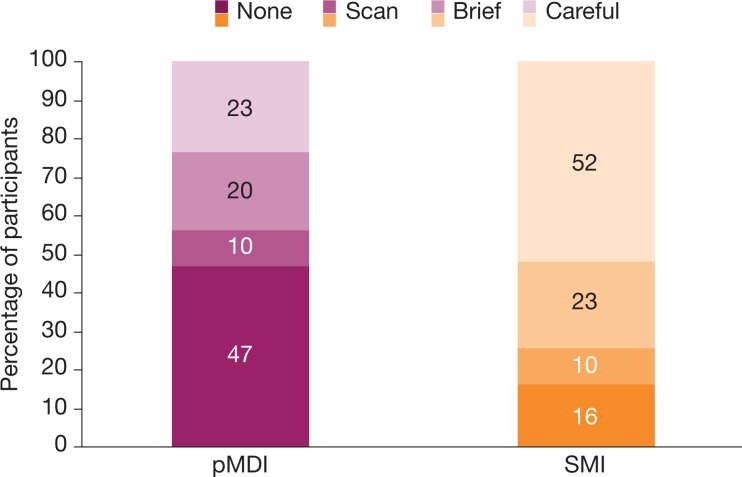
A large proportion of SMI participants (52%) made careful use (i.e. reading each step) of the instructions, compared to 23% of pMDI participants (Figure 2). Nearly half (47%) of the pMDI participants did not use the instructions at all, compared with 16% of SMI participants; these five participants were all experienced in using the SMI.
Figure 2.
Use of instructions. Careful, apparently reading each step; brief, scanning each step and reading some; scan, scanning some steps but ignoring many, with the instructions for use still folded; none, no use at all. SMI total of 101% is a data-rounding artifact. pMDI: pressurized metered dose inhaler; SMI: soft mist inhaler.
Overall task performance error frequency
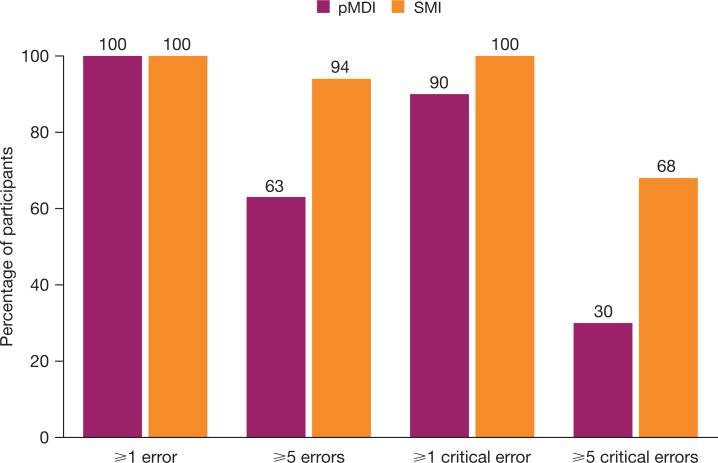
Task performance errors were common with both devices. No participants made no errors and only three (all pMDI) made no critical errors (Figure 3). Overall, 79% (48 of 61) of participants made ≥5 task performance errors (SMI 94%; pMDI 63%) and 49% (30 of 61) made ≥5 critical errors, with fewer pMDI users making ≥5 critical errors (30%), than SMI users (68%). Observed device handling errors are summarized in Table 3, with critical errors highlighted in bold.
Figure 3.
Frequency of errors and critical errors. pMDI: pressurized metered dose inhaler; SMI soft mist inhaler.
Table 3.
Significant and critical device handling errors with the pMDI and SMI.
| Task steps | Proportion of failed tasks (% (n/N))a | ||
|---|---|---|---|
| Device-independent errors | Phase | pMDI | SMI |
| Recognize device is empty (by dose indicator) | Check device | 13 (4/30) | 26 (8/31) |
| Recognize expiry date is exceeded (by expired date on label) | Check device | 40 (12/30) | 34 (10/29) |
| Recognize reorder date (by red field in dose indicator) | Check device | 7 (2/30) | 35 (11/31) |
| Recognize the need to reprime | Repriming | 40 (12/30) | 27 (8/30) |
| Prime device, at least one visible plume | Install cartridge/prime | 3 (1/30) | 29 (9/31) |
| Exhale before inhalation | Routine dosing | 56 (28/50) | 73 (38/52) |
| Close lips around mouthpiece | Routine dosing | 8 (4/50) | 2 (1/52) |
| Inhale slowly b through mouthpiece of device | Routine dosing | 80 (40/50) | 79 (41/52) |
| Activate the device or pressing the button | Routine dosing | 0 (0/50) | 15 (8/52) |
| Hold breath for more than 5 seconds | Routine dosing | 64 (32/50) | 64 (32/50) |
| Repeat inhalation (two inhalations) | Routine dosing | 33 (10/30) | 4 (1/26) |
| Errors applicable to the pMDI only | |||
| Shaking the device | Routine dosing | 40 (20/50) | N/A |
| Hold device correctly during inhalation | Routine dosing | 0 (0/50) | N/A |
| Pressing canister (activating device) only once per inhalation | Routine dosing | 20 (10/50) | N/A |
| Errors applicable to the SMI only | |||
| Remove clear base | Install cartridge/prime | N/A | 0 (0/31) |
| Fully insert device cartridge | Install cartridge/prime | N/A | 81 (25/31) |
| Replace clear base | Install cartridge/prime | N/A | 10 (3/31) |
| Inadvertent activations while priming | Install cartridge/prime | N/A | 32 (10/31) |
| Correct device orientation within 45° of upright during rotation of base | Routine dosing | N/A | 66 (37/56) |
| Rotate device base half a turn until a “click” is heard | Routine dosing | N/A | 30 (17/57) |
| Inadvertent activation during routine dosing | Routine dosing | N/A | 29 (9/31) |
| Inadvertent activations (total) | Routine dosing | N/A | 55 (17/31)c |
N: total number of attempts; n: number of failed attempts; N/A: not applicable; pMDI: pressurized metered dose inhaler; SMI: soft mist inhaler. Steps in italics considered significant, not critical. Critical errors highlighted in bold.
a Participants were supposed to perform some tasks twice. Hence, the total number of attempts varied between tasks.
b Long inhalation lasting >2 seconds.
cTotal number of participants who inadvertently activated the SMI during study. Some participants may have activated the device during priming alone, during dosing alone, or during priming and dosing. In each of these cases, this would only count as a single failure of the task, therefore “inadvertent activations [total]” may doesn’t necessarily equal “inadvertent activations while priming” plus “inadvertent activation during routine dosing.”
pMDI and SMI task performance errors
Device checking
Around a third of participants overall failed to recognize that a device had expired (40 and 34% for pMDI and SMI, respectively), including six participants who correctly identified the expiry date but decided the device could be used anyway. Most patients (pMDI 93%; SMI 65%) recognized the need to reorder. More than 70% of participants for both devices recognized empty devices.
Cartridge installation and device priming
Installing the SMI cartridge was an error-prone task; 25 of the 31 SMI participants failed the task step “inserting the SMI cartridge.” Of the six participants who successfully installed the SMI cartridge, three were current SMI users. Additionally, SMI participants did not hold the SMI in the correct orientation (within 45° from vertical) when rotating the base for two-thirds of attempts, possibly as it is easier to rotate the device when it was held horizontally.
Nine SMI participants and one pMDI participant failed to prime the device with at least one visible plume. Failure to prime with at least one visible plume was considered critical. Based on the instructions for use, patients should prime both devices four times. A further 18 pMDI participants and 20 SMI participants failed to prime the device four times. During priming, 10 SMI participants inadvertently activated the device.
Routine dosing
Participants did not shake the pMDI prior to use for 40% of inhalations, stating that they regarded it as unnecessary or that they forgot to do it. For both devices, most participants failed to fully exhale before placing the device in their mouth. One participant exhaled through the pMDI.
Around 80% of pMDI and SMI participants failed to inhale slowly through the device for >2 seconds. A considerable proportion reported that they were unable to do so as a physical consequence of disease. All pMDI participants held the pMDI correctly during inhalation and all activated the device during inhalation. However, five SMI participants failed to activate the device correctly during inhalation on a total of eight attempts. This included two participants who activated the SMI by holding the button down and rotating the base, while the device was in their mouth. On 20% of occasions, the pMDI participants activated the pMDI more than once per inhalation. Multiple pMDI activations seemed deliberate and were reported as either habitual or trained behavior.
On 64% of occasions, participants failed to perform the recommended 5-second breath hold after inhalation. Typically, participants held their breath for 1–4 seconds.
A high number of pMDI participants (33%) failed to repeat the inhalation because they had activated the device twice during the first inhalation. During dosing, nine SMI participants inadvertently activated the device.
Discussion
This qualitative, exploratory study, which assessed the device handling and usability of a pMDI and an SMI in inhaler-experienced patients with COPD, found that overall task performance was generally poor and errors were common with both devices. Findings mirror previous studies showing that inhaler technique errors are common with all device types, including DPIs, pMDIs, and SMIs.2,3 All participants made errors, only three participants made no critical errors, and nearly all made multiple errors. While critical errors were more common with the SMI than with the pMDI (30% of pMDI users made ≥5 critical errors compared with 68% of SMI users), many errors with the SMI occurred during the installation of the cartridge (25 of 31 participants failed to insert the cartridge correctly), a task step that was not required for the pMDI. While this installation and priming phase was the most error-prone phase for the SMI, it required a higher number of task steps for the SMI compared to the pMDI. Overall, due to the different designs and complexities of these devices, the number of errors in this study was not evenly balanced between devices; 9 of 14 errors assessed for the pMDI and 11 of 19 errors assessed for SMI were considered critical. Therefore, the opportunity for error may have been greater for the SMI than for the pMDI, both for the overall number of task errors and for the critical errors. In addition, only 8 of 31 participants (26%) in the SMI group were a past or current SMI user, compared to 27 of 30 of participants (90%) in the pMDI group who were past or current pMDI users. Hence, the higher error rate reported by SMI participants may at least, in part, be linked to previous device experience.
All study participants were experienced inhaler users and experience was balanced in study groups: in both groups, approximately 90% of participants had experience with a pMDI, and approximately 25% had experience in using a SMI. Therefore, the high error rate observed in both study groups highlights the need for regular review of technique and continued training and education about correct inhaler device use for patients with COPD, which is also emphasized in current GOLD 2017 recommendations.1 As many participants did not carefully read the instructions for use provided, even for an unfamiliar device, healthcare professionals should continue to encourage using instructions and other written materials and provide user demonstrations and personalized training. Healthcare professionals should also continuously assess inhaler technique, even when patients feel confident their technique is correct and retrain patients as required. The less frequent use of instructions by participants using the pMDI compared to the SMI could suggest that pMDI participants felt confident using the device. However, this study found that these participants still exhibited a high number of handling errors.
Further research is needed to determine how well patients respond to device technique training, and whether patients are likely to improve more rapidly with training on one device or the other. The lower number of participants making ≥5 critical errors using the pMDI compared to the SMI suggests that it may prove easier to train patients in the correct use of a pMDI. A recent study in patients with asthma or COPD found that fewer attempts were needed to successfully use inhalers requiring fewer tasks steps prior to device actuation.23
As recommended by GOLD, maintenance treatment of COPD should be individualized and guided by multiple factors, including patient response, preference, and ability to use various delivery devices;1 it is essential that physicians prescribe an inhaler that patients are able and willing to use.13,24 Hence, availability of COPD treatments in multiple devices would allow physicians to consider individual patient preferences, needs, and physical abilities when selecting treatments. For example, fixed-dose combinations of long-acting muscarinic antagonists/long-acting β2-agonists have, until recently, only been available as DPIs25–27 but are now also available as pMDIs and SMIs.20,21
Potential study limitations include that not all tasks associated with pMDI and SMI use were assessed, such as device unpacking, cleaning, storing, and disposal. In a real-world setting, patients may use a pMDI with a spacer, but handling errors with a spacer were not explored in our study. No statistical analyses were planned or performed, which is typical for this type of exploratory, qualitative study. Due to the relatively small sample size, the groups were slightly imbalanced for COPD severity and a meaningful analysis of results in relation to age, gender, or disease severity was not possible. The research setting was dissimilar to the home environment, which may have affected task performance. However, this qualitative study design is common for exploring inhalation devices and allowed discussion with participants to explore the root causes of observed errors.
It should also be noted that for both devices, drug release from the device may be an important factor in patient behavior and device technique, and the absence of the sensation of medicine release during the simulated inhalation may have affected device operation by participants. Other detailed aspects such as the start, strength, and duration of inhalation; the effectiveness of inhalation and exhalation; tongue position during inhalation; the effectiveness of lip seal on the mouthpiece; and the precise assessment of the coordination of inhalation with actuation of the empty pMDI and SMI devices were not evaluated but remain important for effective drug delivery through inhaler devices.
For future studies, it would also be of interest to explore the potential impact of individual critical errors on treatment and clinical efficacy in the real world. As it is not clear whether critical handling errors affect adherence in the real world, it would be interesting to investigate a potential correlation between patients, critical errors, frequency of errors and adherence to treatment, or willingness to adhere to treatment.
Conclusion
The findings of this observational, explorative study highlight that regardless of the inhaler device used and previous device experience, key aspects of the routine patient care in COPD should be patient training, education, and continuous retraining on the correct, effective use of the inhaler device, tailored to the needs of the patient, as emphasized by current GOLD treatment recommendations.
Supplemental material
RWE_Device_Handling_Study_MS_SUPPLEMENTARY_MATERIAL for Inhaler usability of a pressurized metered dose inhaler and a soft mist inhaler in patients with COPD: A simulated-use study by Bo Ding, Shahid Siddiqui, Michael DePietro, Gunilla Petersson, and Ubaldo J Martin in Chronic Respiratory Disease
Acknowledgements
The authors would like to thank Martin Bontoft and Claire Young of Team Consulting Limited, UK, for their involvement in development and implementation of the study protocol. The authors also thank all the patients and their families and the team of investigators involved in the study. Medical writing support, under the direction of the authors, was provided by Siobhán Hoy of CMC CONNECT, a division of McCann Health Medical Communications Ltd (formerly known as McCann Complete Medical Communications Ltd), UK, which was funded by AstraZeneca, Cambridge, UK, in accordance with Good Publication Practice (GPP3) guidelines.28
Authors’ note: BD, SS, MDP, and UJM were involved in the study design. BD, SS, MDP, GP, and UJM were involved in the protocol review, the study interpretation, and the manuscript preparation. SS was also involved in collaboration during vendor selection, site selection, Web-based, remote review of the study conduct, and budget sponsorship.
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: BD, SS, GP and UJM are employees of AstraZeneca. MDP is a former employee of AstraZeneca.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by AstraZeneca.
ORCID iD: Michael DePietro  http://orcid.org/0000-0002-8729-2530
http://orcid.org/0000-0002-8729-2530
Supplemental material: Supplementary material for this article is available online.
References
- 1. Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management and Prevention of COPD. 2017. http://www.goldcopd.org (accessed 8 September 2017).
- 2. Molimard M, Raherison C, Lignot S, et al. Chronic obstructive pulmonary disease exacerbation and inhaler device handling: real-life assessment of 2935 patients. Eur Respir J 2017; 49: 1601794. [DOI] [PubMed] [Google Scholar]
- 3. Chorão P, Pereira AM, Fonseca JA. Inhaler devices in asthma and COPD—an assessment of inhaler technique and patient preferences. Respir Med 2014; 108: 968–975. [DOI] [PubMed] [Google Scholar]
- 4. Arora P, Kumar L, Vohra V, et al. Evaluating the technique of using inhalation device in COPD and bronchial asthma patients. Respir Med 2014; 108: 992–998. [DOI] [PubMed] [Google Scholar]
- 5. Khassawneh BY, Al-Ali MK, Alzoubi KH, et al. Handling of inhaler devices in actual pulmonary practice: metered-dose inhaler versus dry powder inhalers. Respir Care 2008; 53: 324–328. [PubMed] [Google Scholar]
- 6. Pothirat C, Chaiwong W, Phetsuk N, et al. Evaluating inhaler use technique in COPD patients. Int J Chron Obstruct Pulmon Dis 2015; 10: 1291–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Melani AS, Bonavia M, Cilenti V, et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med 2011; 105: 930–938. [DOI] [PubMed] [Google Scholar]
- 8. Lewis A, Torvinen S, Dekhuijzen PN, et al. The economic burden of asthma and chronic obstructive pulmonary disease and the impact of poor inhalation technique with commonly prescribed dry powder inhalers in three European countries. BMC Health Serv Res 2016; 16: 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Melzer AC, Ghassemieh BJ, Gillespie SE, et al. Patient characteristics associated with poor inhaler technique among a cohort of patients with COPD. Respir Med 2017; 123: 124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bartolo K, Balzan M, Schembri EL, et al. Predictors of correct technique in patients using pressurized metered dose inhalers. BMC Pulm Med 2017; 17: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Westerik JA, Carter V, Chrystyn H, et al. Characteristics of patients making serious inhaler errors with a dry powder inhaler and association with asthma-related events in a primary care setting. J Asthma 2016; 53: 321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dahl R, Backer V, Ollgaard B, et al. Assessment of patient performance of the HandiHaler compared with the metered dose inhaler four weeks after instruction. Respir Med 2003; 97: 1126–1133. [DOI] [PubMed] [Google Scholar]
- 13. Laube BL, Janssens HM, de Jongh FH, et al. What the pulmonary specialist should know about the new inhalation therapies. Eur Respir J 2011; 37: 1308–1331. [DOI] [PubMed] [Google Scholar]
- 14. Anderson P. Use of respimat soft mist inhaler in COPD patients. Int J Chron Obstruct Pulmon Dis 2006; 1: 251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brand P, Hederer B, Austen G, et al. Higher lung deposition with respimat soft mist inhaler than HFA-MDI in COPD patients with poor technique. Int J Chron Obstruct Pulmon Dis 2008; 3: 763–770. [PMC free article] [PubMed] [Google Scholar]
- 16. ZuWallack R, De Salvo MC, Kaelin T, et al. Efficacy and safety of ipratropium bromide/albuterol delivered via Respimat inhaler versus MDI. Respir Med 2010; 104: 1179–1188. [DOI] [PubMed] [Google Scholar]
- 17. Kilfeather SA, Ponitz HH, Beck E, et al. Improved delivery of ipratropium bromide/fenoterol from Respimat Soft Mist Inhaler in patients with COPD. Respir Med 2004; 98: 387–397. [DOI] [PubMed] [Google Scholar]
- 18. Iacono P, Velicitat P, Guemas E, et al. Improved delivery of ipratropium bromide using Respimat (a new soft mist inhaler) compared with a conventional metered dose inhaler: cumulative dose response study in patients with COPD. Respir Med 2000; 94: 490–495. [DOI] [PubMed] [Google Scholar]
- 19. British Healthcare Business Intelligence Association. Legal and Ethical Guidelines for Healthcare Market Research: Your essential guide. 2017. https://www.bhbia.org.uk/guidelines/legalandethicalguidelines.aspx (accessed 20 September 2017).
- 20. AstraZeneca Pharmaceuticals LP. Bevespi Aerosphere™ Prescribing Information. 2016. http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/208294s000lbl.pdf (accessed 21 September 2017).
- 21. Boehringer Ingelheim Pharmaceuticals, Inc. STIOLTO™ RESPIMAT® Prescribing Information 2015. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/206756Orig1s000lbl.pdf (accessed 3 July 2018).
- 22. US Food and Drug Administration. Applying Human Factors and Usability Engineering to Medical Devices; Guidance for Industry and Food and Drug Administration Staff. 2016. https://www.fda.gov/downloads/MedicalDevices/…/UCM259760.pdf (accessed 19 June 2017).
- 23. Dal Negro RW, Povero M. Acceptability and preference of three inhalation devices assessed by the handling questionnaire in asthma and COPD patients. Multidiscip Respir Med 2016; 11: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Braido F, Chrystyn H, Baiardini I, et al. “Trying, but failing”—the role of inhaler technique and mode of delivery in respiratory medication adherence. J Allergy Clin Immunol Pract 2016; 4: 823–832. [DOI] [PubMed] [Google Scholar]
- 25. GlaxoSmithKline. Anoro Ellipta Summary of Product Characteristics. 2017. http://www.medicines.org.uk/emc/medicine/28949 (accessed 8 September 2017).
- 26. Novartis Europharm Limited. Ultibro® Breezhaler Summary of Product Characteristics. 2016. https://www.medicines.org.uk/emc/medicine/29533 (accessed 21 September 2017).
- 27. AstraZeneca AB. Duaklir Genuair Summary of Product Characteristics. 2017. https://www.medicines.org.uk/emc/medicine/29652 (accessed 21 September 2017).
- 28. Battisti WP, Wager E, Baltzer L, et al. Good publication practice for communicating company-sponsored medical research: GPP3. Ann Int Med 2015; 163: 461–464. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
RWE_Device_Handling_Study_MS_SUPPLEMENTARY_MATERIAL for Inhaler usability of a pressurized metered dose inhaler and a soft mist inhaler in patients with COPD: A simulated-use study by Bo Ding, Shahid Siddiqui, Michael DePietro, Gunilla Petersson, and Ubaldo J Martin in Chronic Respiratory Disease