Abstract
Primary ciliary dyskinesia (PCD) is a rare disease causing motile cilia dysfunction, recurrent airway infection, and bronchiectasis. Airway infection management strategies are borrowed from cystic fibrosis. The aim of this study is to describe the management of airway infection with Pseudomonas aeruginosa (PA) in children and adults with PCD across European centers. An online survey questionnaire was sent electronically using SurveyMonkey® to 55 PCD centers in 36 European countries. Fifty-two responded from 43 centers in 26 countries, a response rate of 70%. Most (89%) countries did not have written guidelines for PCD management. Airway sampling for infection detection at each clinic visit was more likely when follow-up was frequent. Eighty-seven percent of centers chose to treat the first PA isolate, most prescribing combined oral ciprofloxacin and inhaled colistimethate sodium (43%, n = 18). The preferred treatment for chronic infection with PA was nebulized colistimethate in 51% (n = 22). In summary, considerable variation exists across European centers in the frequency of patient follow-up and airway sampling for infection, treatment goals, and the management of PA infection. Few centers had written guidelines for PCD management. Clinical trials to determine optimal treatment of PA in PCD patients are urgently needed.
Keywords: Primary ciliary dyskinesia, Pseudomonas aeruginosa, children, adults, Europe
Introduction
Primary ciliary dyskinesia (PCD) is a rare inherited disease of motile cilia dysfunction causing repeated respiratory tract infections and the eventual development of bronchiectasis. The airway bacterial diversity in PCD resembles that of non-cystic fibrosis bronchiectasis.1 Non-typeable Haemophilus influenzae and Moraxella catarrhalis are the predominant airway pathogens in childhood and adolescence.1,2 Chronic infection with Pseudomonas aeruginosa (PA) becomes more prevalent with age, affecting at least 5% of children and 39% of adults, and is associated with poorer lung function and delayed diagnosis.1–5 The European Cystic Fibrosis Society has published consensus guidelines for standards of care in cystic fibrosis (CF),6 including optimal recommendations for the prevention and treatment of PA infection. Two recently published expert reviews of clinical care in PCD7,8 acknowledged the lack of evidence for the management of airway infection. The European Respiratory Society has now published guidelines for the management of adult bronchiectasis including PCD,9 concluding that PA eradication may positively influence clinical outcomes. However, to date, management of airway infection including PA in PCD has been haphazard and extrapolated from CF practice without evidence of efficacy.10 In order to understand current management practices in PCD, we conducted, on behalf of BEAT-PCD, an online questionnaire-based survey of European PCD centers with the aim of describing the various regimens employed in the treatment of acute, intermittent, and chronic PA infection.
Methods
European PCD centers in 36 countries were identified through their membership of BEAT-PCD and by searching the European Respiratory Society database of members. Europe was defined according to the United Nations geoscheme for Europe11 with the addition of Armenia, Cyprus, and Turkey. Survey questionnaires were sent electronically using SurveyMonkey (SurveyMonkey®, San Mateo, California, USA). Reminders were sent twice to nonresponders. The methodology and survey questionnaire were adapted from a survey of CF treatment practices performed by Elborn et al., with their consent, and included 29 open and directed questions.12 The survey questionnaire, available in the Online Supplementary material, was completed by PCD specialist clinicians. Intermittent and chronic PA infection were defined according to modified Leeds criteria:1,13
chronic infection, when more than 50% of the preceding 12 months’ cultures were positive for PA;
intermittent infection, when 50% or less of the preceding 12 months’ cultures were positive for PA;
free of infection, when no growth has occurred in the lungs in the previous 12 months.
Statistical analysis was performed using IBM SPSS Statistics 22.
Survey replies were analyzed manually due to the qualitative nature of the survey. Five centers treating both children and adults gave dual replies, each corresponding to the care of children and adults, respectively. These replies were weighted to account for 50% of the center’s answer in questions not directly allocated to the care of either children or adults. Data are reported as the number and percentages of centers answering each question. Weighting of centers means that center numbers are reported in decimal points. χ2 test with linear-by-linear association was used to test for linear trends associated with center size.
Results
Center characteristics
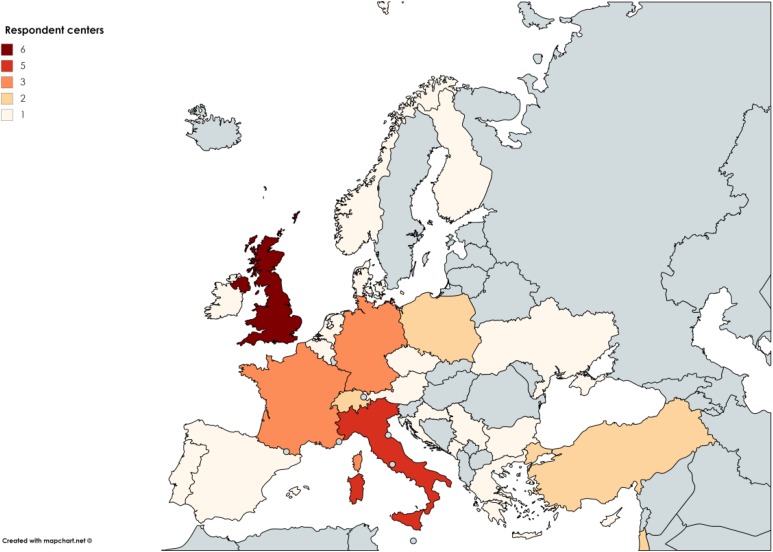
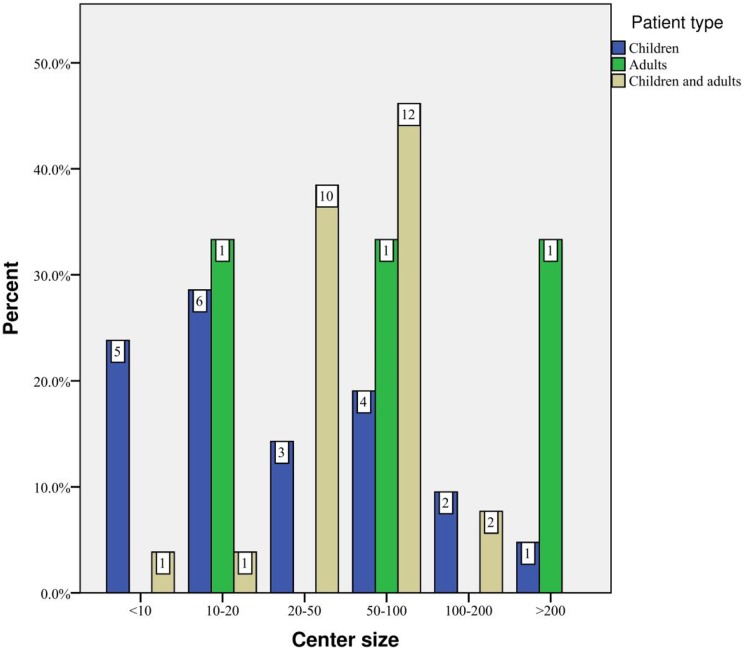
Seventy-four survey questionnaires were sent to 55 European PCD centers. There were 52 respondents from 43 centers in 26 countries (Figure 1), a response rate of 70.3%. Most responding centers cared for children (51.2%, n = 22) or children and adults (41.9%, n = 18), with 7.0% (n = 3) caring for adults only. There was considerable variation in the size of “children only centers” and 50.0% (n = 11) had less than 20 patients. Six centers cared for at least 50 children. Most centers (83.3%, n = 15) caring for both children and adults had between 20 and 100 patients (Figure 2), while the size of adult-only centers ranged from more than 10 to more than 200 patients. Four respondents’ replies were discarded since they duplicated results already received from the same center. A total of 48 replies were included. Two centers did not report patient numbers.
Figure 1.
The number of participating centers in each European country.
Figure 2.
The distribution of patient numbers according to center type.
PCD management
Most countries (88.5%, n = 23) lacked written guidelines for PCD management. There was however within-country disagreement, with 71.4% (n = 5) of UK, 25.0% (n = 1) of German, 50.0% (n = 1) of Israeli, and 25.0% (n = 1) of French respondents answering “yes” to this question. Only three countries, Ukraine (one center), United Kingdom, and France, have written guidelines for the complications of PCD, but again there was disagreement between respondents with 83.3% (n = 5) of UK and 75.0% (n = 3) of French centers answering “no” to this question. The majority of pediatric centers (54.5%, n = 12) saw their patients in outpatient clinics every 1–3 months, in contrast to adult-only centers where patients were seen at a greater than 3-month interval. In mixed pediatric and adult centers, around 75% of patients were seen every 1–3 months. Center size did not impact follow-up frequency (χ 2(1) = 1.005, p = 0.316) but was associated with delay in hospital admission for treatment of a respiratory exacerbation (χ 2(1) = 6.212, p = 0.013).
Airway infection monitoring
While nearly all centers cultured sputum to detect bacterial airway infection, a cough swab was preferred over laryngeal aspirate for patients, including adults, who could not expectorate (Table 1). Most (81.8%, n = 18) pediatric centers did not perform laryngeal aspirate sampling. Only 26.7% (n = 11.5) of all centers cultured nasal lavage or nasal swab specimens for bacteria. Seventy-five percent of all centers obtained samples for bacterial culture at every clinic visit. Almost all (92.2%, n = 23.5) centers seeing patients every 1–3 months took a sample at every visit, while fewer (54.5%, n = 9) centers seeing patients at longer intervals did so. All centers performed routine in vitro antibiotic sensitivity testing but not synergy testing (available in 32.6%, n = 14). A small percentage, 5.8% (n = 2.5), did not base antibiotic prescribing on culture results. Molecular diagnostic methods for gram-negative organisms were available in 72.1% (n = 31) of centers. All centers, with the exception of one, routinely cultured for PA but considerably fewer, 22.1% (n = 9.5), measured precipitating IgG antibodies against PA.
Table 1.
Routine sampling to detect airway infection.
| Age group | Sputum, N (%) | Laryngeal aspirate, N (%) | Cough swab, N (%) | Nasal swab or lavage, N (%) |
|---|---|---|---|---|
| Children | 21.0 (95.5) | 4.0 (18.2) | 16.0 (72.7) | 5.0 (22.7) |
| Adults | 3.0 (100) | 0 | 0 | 0 |
| Children and adults | 17.5 (97.2) | 4.0 (22.2) | 8.5 (47.2) | 6.5 (36.1) |
| Totala | 41.5 (96.5) | 8.0 (18.6) | 24.5 (57.0) | 11.5 (26.7) |
a Number (%) of centers.
Management of airway infection with PA
Most (87.2%, n = 37.5) centers prescribed antibiotics for newly acquired PA and of these, 79.5% opted for inhaled treatment. The indication for treatment differed between centers; two of the three adult centers treated PA infection only if lung function was deteriorating, in contrast to 72.7% (n = 16) of pediatric centers, which would treat irrespective of lung function. The most commonly prescribed regimen for PA eradication was a combination of oral ciprofloxacin and inhaled colistimethate sodium (41.9%, n = 18); 19.8% (n = 8.5) used oral ciprofloxacin alone, 16.3% (n = 7) used inhaled tobramycin alone, and 14.0% (n = 6) used a combination of oral ciprofloxacin and inhaled tobramycin (Table 2). Intermittent growth of PA was treated differently, with more centers choosing to use inhaled antibiotics alone (Table 3).
Table 2.
Antibiotic regimens used for the eradication of PA infection.
| Drug combination | Inhaled tobramycin, N (%) | Inhaled colistimethate, N (%) | Oral ciprofloxacin, N (%) | Other drug, N (%) | Total,a N (%) |
|---|---|---|---|---|---|
| Oral ciprofloxacin | 6.0 (14.5) | 18.0 (43.4) | 0 | 1.0 (2.4) | 25.0 (60.2) |
| No ciprofloxacin | 7.0 (16.9) | 0 | 8.5 (20.5) | 1.0 (2.4) | 16.5 (39.8) |
| Totala | 13.0 (31.3) | 18.0 (43.4) | 8.5 (20.5) | 2.0 (4.8) | 41.5 (100) |
PA: Pseudomonas aeruginosa.
a Number (%) of centers.
Table 3.
Antibiotic regimens for the treatment of intermittent infection with PA.
| Drug combination | Inhaled tobramycin, N (%) | Inhaled colistimethate, N (%) | Oral ciprofloxacin, N (%) | Other drug, N (%) | Total,a N (%) |
|---|---|---|---|---|---|
| Oral ciprofloxacin | 5.0 (12.8) | 8.0 (20.5) | 0 | 1.0 (2.6) | 14.0 (35.9) |
| No ciprofloxacin | 9.0 (23.1) | 6.5 (16.7) | 9.5 (24.4) | 0 | 25.0 (64.1) |
| Totala | 14.0 (35.9) | 14.5 (37.2) | 9.5 (24.4) | 1.0 (2.6) | 39.0 (100) |
PA: Pseudomonas aeruginosa.
a Number (%) of centers.
The duration of antibiotic treatment for eradication of PA infection varied, with most centers choosing either oral ciprofloxacin for 2 or 4 weeks and inhaled antibiotics for either 1 or 3 months (Table 4). Around 60% of pediatric centers opted for a shorter 1-month duration of inhaled treatment, while 54% of mixed pediatric and adult centers treated for 3 months.
Table 4.
Duration of inhaled and oral antibiotic treatment for the eradication of PA infection.
| Duration | Inhaled antibiotic (months) | ||||
|---|---|---|---|---|---|
| Oral ciprofloxacin (weeks) | 1, N (%) | 2, N (%) | 3, N (%) | 6, N (%) | Total,a N (%) |
| 2 | 7.0 (18.9) | 1.0 (2.7) | 9.5 (25.7) | 0 | 17.5 (47.3) |
| 3 | 2.5 (6.8) | 2.0 (5.4) | 2.0 (5.4) | 0 | 6.5 (17.6) |
| 4 | 5.5 (14.9) | 0 | 4.0 (10.8) | 1.0 (2.7) | 10.5 (28.4) |
| 6 | 0 | 0 | 1.0 (2.7) | 0 | 1.0 (2.7) |
| 13 | 0 | 0 | 1.5 (4.1) | 0 | 1.5 (4.1) |
| Totala | 15.0 (40.6) | 3.0 (8.1) | 18.0 (48.6) | 1.0 (2.7) | 37.0 (100) |
PA: Pseudomonas aeruginosa.
a Number (%) of centers.
The preferred regimen for treatment of recurring PA infection after attempted eradication was intravenous (IV) antibiotics either alone (15.1%, n = 6.5) or in combination (31.4%, n = 13.5) with inhaled antibiotics. Therapy with IV ceftazidime and tobramycin was more common than a carbapenem combination and 12.8% (n = 5.5) of centers opted for single therapy with either IV ceftazidime or a carbapenem. Treatment duration of at least 10 days was almost universal (96%). Fewer centers (20.9%, n = 9) chose a combination of nebulized colistimethate and oral ciprofloxacin over IV therapy.
Chronic airway infection with PA
For most centers (86.0%, n = 37), the most important therapeutic goal in patients with chronic PA infection was improvement or stabilization of lung function; eradication of infection and reduction of IV antibiotic need were the least important aims. Nebulized colistimethate was used by 51.2% (n = 22) of centers, tobramycin in 33.7% (n = 14.5), and gentamicin in 5.8% (n = 2.5). For chronic PA infection with exacerbation, the most common choice was continuous inhaled antibiotic combined with oral ciprofloxacin (50.0%, n = 21.5). A quarter of respondents were treated with continuous inhaled antibiotic and monotherapy with either IV carbapenem or ceftazidime. A further 25% would treat with continuous inhaled antibiotic combined with either IV carbapenem or ceftazidime and IV tobramycin. Few centers (9.3%, n = 4) used continuous inhaled antibiotic combined with regular 3 monthly IV antibiotic treatment.
Discussion
Lung function in PCD patients is affected to a similar degree to pancreatic sufficient CF patients, with a similar rate of decline with age and similar prevalence of PA infection.14 However, the impact of chronic PA infection in PCD is unclear, being associated with poorer lung function in some studies4,5,15 but not all.14,16 Early aggressive treatment aimed at eradicating PA infection has been successful in preventing chronic infection and reducing the rate of infectious exacerbations in CF17 and non-CF bronchiectasis18 with higher eradication rates in the latter17 and preservation of lung function in CF.19 Although prophylactic treatment for PA infection in children with PCD has been recommended,10 there are no published data on outcomes of inhaled, oral, or IV treatment for acute, intermittent, or chronic PA infection in either adult or pediatric PCD patients. To the best of our knowledge, the results of this survey describe for the first time the various regimens adopted by different European centers for the treatment of PA infection in PCD.
Summary of main findings
PCD management
Most centers participating in this survey had no written guidelines for the management of PCD or its complications and accordingly, there was considerable variation in basic PCD management such as the frequency of follow-up visits and the frequency of sampling of airway secretions for bacterial culture. Children with PCD were followed up more frequently than adults who were managed in adult-only centers. Given the age-related increasing prevalence of PA infection and deteriorating lung function,4,5,15 closer follow-up of adults has the potential to influence these outcomes.
Airway infection monitoring
The ability to detect infection is related to the frequency and type of sampling of airway secretions. In this survey, 57% of centers used cough swabs while only 18.6% chose laryngeal suction, despite the poor sensitivity of cough swabs in detecting lower airway infection.20 Laryngeal samples obtained by suction or swabs may better reflect lower airway infection21 but there are no comparative studies of the utility of cough swabs versus laryngeal aspirates in the detection of lower airway infection in CF and PCD. Moreover, current European management guidelines for children with PCD recommend cough swabs for infection monitoring despite their poor sensitivity.10 Centers that followed up patients less frequently were less likely to culture airway secretions at each clinic visit and fewer than 30% of all centers routinely cultured nasal secretions. This may be relevant since a small study of PCD patients aged 11–50 years undergoing endoscopic sinus surgery found simultaneous infection with PA in the lungs and paranasal sinuses in two-thirds of patients, suggesting that the upper airways may function as a reservoir for bacterial infection of the lower airways.22 Nonetheless, it was encouraging to see that all centers routinely performed in vitro antibiotic sensitivity testing and that more than 90% of centers based antibiotic choice on the results, thus reducing the risk of antibiotic resistance developing.
Management of PA airway infection
All centers except one routinely cultured for PA, but few measured specific precipitating IgG antibodies, despite this being a highly sensitive and specific test for infection with mucoid strains of PA.23 In addition to children being followed up at more frequent intervals than adults, children were more likely than adults to be treated for newly acquired PA. In fact, centers treating adults tended to attempt PA eradication only if lung function was deteriorating or after at least two positive cultures. In a relatively large study of factors contributing to eradication failure of PA infection in CF, the likelihood of successful eradication increased by 17% for each additional sputum sample before the first isolation, while eradication failure increased by 1.3% for each month of delayed CF diagnosis.24 Thus, both sampling frequency and age at diagnosis are likely important factors in the successful eradication of PA infection in CF. If the same is true for PCD, which in Europe has a median age at diagnosis of 3.5 years for those with situs inversus and 5.8 years for those without,25 then earlier diagnosis and better microbiological surveillance and antibiotic treatment may delay the onset of chronic PA infection. No single eradication regimen has been shown to be superior, thus options include 28 days of inhaled tobramycin and up to 3 months of nebulized colistimethate and oral ciprofloxacin.6 More than half the centers surveyed opted for combined treatment with oral ciprofloxacin and either nebulized colistimethate or tobramycin with fewer than 20% choosing monotherapy with inhaled tobramycin, usually for 1 or 3 months. A similar percentage also opted for monotherapy with either IV ceftazidime or a carbapenem for the treatment of PA eradication failure. The choice of monotherapy may not be optimal given the possible synergy between ceftazidime and tobramycin in the treatment of PA 26 and the implication of ceftazidime monotherapy in an outbreak of resistant PA in a children’s CF clinic.27
In adult-only and the majority of pediatric centers, there was a preference for stabilization of lung function over eradication of PA as the primary treatment goal. Centers treating adults only were less likely to attempt eradication by treating the first isolation of PA and followed up their patients at longer intervals, suggesting that adults with PCD may be managed less aggressively than children. Although the number of centers treating adults only was small, the patient total in these centers exceeded 250.
Strengths and limitations
Although the survey response rate was 70%, we were unable to identify all PCD centers in Europe and cannot therefore give a complete overview of PA treatment strategies. Nonetheless, this survey likely reflects current practice in the 26 European countries represented. One country, Lithuania, declined to participate in the survey because patients with PCD are classified as having CF in order to receive free medicines and thus PCD care is not systematized. In four countries, Belgium, Bulgaria, Turkey, and Ukraine, inhaled antibiotics are rarely used in PCD because costs are reimbursed only for CF patients, a factor that impacted respondents’ prescribing practice. We did not ascertain whether patients were treated in centers with access to a multidisciplinary PCD team, a desirable component of specialist PCD care, or within CF and respiratory clinics, factors that may contribute to variation in airway sampling across centers.
Implications for future research
This survey of the management of PA airway infection in PCD patients shows variation between European centers caring for only adults and those caring for only children in the frequency of follow-up visits, the frequency of airway sampling for microbial culture, treatment goals, and choice and duration of inhaled and IV antibiotic treatment used for PA eradication. Additionally, few centers have written guidelines for the management of PCD and its complications, which is likely to encourage haphazard and inconsistent treatment.7 These differences, which may adversely affect morbidity in PCD patients, can be utilized in the development of urgently needed clinical trials to determine the most effective treatment strategies for the management of newly acquired and chronic PA infection.
Supplemental material
Supplemental Material, On_line_supplement_resubmission_CRD for Variation in treatment strategies for the eradication of Pseudomonas aeruginosa in primary ciliary dyskinesia across European centers by Suzanne Crowley, Mathias Geldermann Holgersen, and Kim Gjerum Nielsen in Chronic Respiratory Disease
Acknowledgements
The authors thank the following adult and pediatric physicians who contributed data for the survey: Ines Azevedo, Central Hospital San João, Porto, Portugal; Angelo Barbato, PCD Regional Center, University General Hospital, Padova, Italy; Mieke Boon, University Hospital Gasthuisberg, Leuven, Belgium; Siobhan Carr, Department of Paediatric Pulmonology, Royal Brompton Hospital, London, UK; Andre Coste, Hôpital Intercommunal de Créteil, France; Suzanne Crowley, Department of Allergic and Lung Diseases, Oslo University Hospital, Oslo, Norway; Ernst Eber, Department of Paediatrics and Adolescent Medicine, University Hospital, Graz, Austria; Virginie Escabasse, Centre Hospitalier Intercommunal de Creteil, France; Amparo Escribano, University Hospital Clinic, Valencia, Spain; Eric Haarman, VU University Medical Center, Amsterdam, Netherlands; Natalyia Haliyash, Ternopil Regional Paediatric Hospital, Ukraine; Isabelle Honore, Hôpital Cochin, Paris, France; Hasnaa Ismail-Koch, Department of Paediatrics, Southampton General Hospital, Southampton, UK; Mark Jorissen, University Hospital, Leuven, Belgium; Andreas Jung, Children’s University Hospital, Zurich, Switzerland; Bulent Karadag, Academic Department of Paediatrics, Marmara University Hospital, Istanbul, Turkey; Priti Kenia, Birmingham Children’s Hospital, UK; Etian Kerem, Hadassah University Hospital, Jerusalem, Israel; Cordula Koerner-Rettberg, University Children’s Hospital at St. Joseph’s Hospital, Bochum, Germany; Romain Lazor, Respiratory Department, Lausanne University Hospital, Switzerland; Barry Linnane, University Hospital, Limerick, Ireland; Michael Loebinger, Royal Brompton Hospital, London, UK; Enrico Lombardi, Paediatric Pulmonary Unit, Meyer University Hospital, Firenze, Italy; Natalie Lorent, Universitaire ziekenhuizen Leuven, Belgium; Jane Lucas, Department of Paediatrics, Southampton General Hospital, Southampton, UK; Heikki Lukkarinen, Turku University Hospital, Finland; Bernard Maitre, Service de Pnuemonologi, Hôpital Intercommunal de Créteil, France; Goran Markovic, Clinical Center of Montenegro, Podorica, Montenegro; Henrik Mazurek, Department of Pulmonology and Cystic Fibrosis, Rabce-Zdròj, Poland; Eduardo Moya, Leeds and Bradford Paediatric PCD Center for North of England, Bradford, England; Huda Mussaffi-Georgy, Schneider Children’s Medical Center, Tel-Aviv, Israel; Kim G Nielsen, Danish PCD & chILD Centre, CF Centre Copenhagen, Paediatric Pulmonary Service, Denmark; Ugur Ozcelik, Department of Paediatric Pulmonology, Hacettepe University Medical Faculty, Ankara, Turkey; Rita Padoan, PCD Regional Center, Brescia, Italy; Ivan Pavic, Children’s Hospital Zagreb, Croatia; Petr Pohunek, Department of Paediatric Pulmonology, University Hospital Motol, Prague, Czech Republic; Mareike Price, Department of Paediatric Pulmonology, Hannover University Medical School, Germany; Kostas Priftis, Children’s Respiratory and Allergy Unit, Attikon University Hospital, Athens, Greece; Alastair Reid, Royal Belfast Hospital for Sick Children, Belfast, UK; Felix Ringhausen, Adult Bronchiectasis and PCD Center, Hannover, Germany; Nisreen Rumann, Department of Paediatrics, Makassed Hospital, Jeruslaem, Palestine; Teresa Salermo, Bambino Gesù Hospital, Roma, Italy; Francesca Santamaria, Department of Paediatric Pulmonology, Fredrico II University, Napoli, Italy; Nico Schwerk, Department of Academic Paediatrics, Hannover, Germany; Deborah Snijders, PCD Center, Department of Women’s and Children’s Health, University of Padova, Italy; Guergana Stoyanova, Children’s University Clinic, Hospital for Lung Diseases, Sofia, Bulgaria; Guillame Thouvenin, Department of Paediatrics, Armand Trousseau Hospital, Paris, France; Claudius Werner, University Hospital Muenster, Germany; Irena Wojsyk-Banaszak, Poznan University of Medical Sciences, Poland; Woolf Walker, PCD Center, Southampton General Hospital, Southampton, UK; Panayiotis Yiallouros, Cyprus PCD Center, Nikosia, Cyprus; and Zorica Zivkovic, Children’s Hospital for Lung Diseases and TB, Medical Center dr Dragisa Misovic, Belgrade, Serbia.
Suzanne Crowley and Kim Gjerum Nielsen are members of BEAT-PCD (Better Experimental Approaches to Treat PCD), COST action BM1407 supported by the EU framework programme Horizon 2020.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Suzanne Crowley  http://orcid.org/0000-0003-2786-8718
http://orcid.org/0000-0003-2786-8718
Mathias Geldermann Holgersen  http://orcid.org/0000-0001-9944-6253
http://orcid.org/0000-0001-9944-6253
Supplemental material: Supplemental material for this article is available online.
References
- 1. Alanin MC, Nielsen KG, von Buchwald C, et al. A longitudinal study of lung bacterial pathogens in patients with primary ciliary dyskinesia. Clin Microbiol Infect 2015; 21: 1093, e1–e7. [DOI] [PubMed] [Google Scholar]
- 2. Maglione M, Bush A, Nielsen KG, et al. Multicenter analysis of body mass index, lung function, and sputum microbiology in primary ciliary dyskinesia. Pediatr Pulmonol 2014; 49: 1243–1250. [DOI] [PubMed] [Google Scholar]
- 3. Noone PG, Leigh MW, Sannuti A, et al. Primary ciliary dyskinesia: diagnostic and phenotypic features. Am J Respir Crit Care Med 2004; 169: 459–467. [DOI] [PubMed] [Google Scholar]
- 4. Shah A, Shoemark A, MacNeill SJ, et al. A longitudinal study characterising a large adult primary ciliary dyskinesia population. Eur Respir J 2016; 48: 297–300. [DOI] [PubMed] [Google Scholar]
- 5. Frija-Masson J, Bassinet L, Honoré I, et al. Clinical characteristics, functional respiratory decline, and follow-up in patients with primary ciliary dyskinesia. Thorax 2017; 72: 154–160. [DOI] [PubMed] [Google Scholar]
- 6. Smyth AR, Bell SC, Bojcin S, et al. European Cystic Fibrosis Society standards of care: best practice guidelines. J Cyst Fibros 2014; 13: S23–S42. [DOI] [PubMed] [Google Scholar]
- 7. Lucas JS, Alanin MC, Collins S, et al. Clinical care of children with primary ciliary dyskinesia. Exp Rev Respir Med 2017; 11: 779–790. [DOI] [PubMed] [Google Scholar]
- 8. Shapiro AJ, Zariwala MA, Ferkol T, et al. Diagnosis, monitoring, and treatment of primary ciliary dyskinesia: PCD foundation consensus recommendations based on state of the art review. Ped Pulmonol 2016; 51: 115–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Polverino E, Geominne PC, McDonnell MJ, et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J 2017; 50: 1700629. [DOI] [PubMed] [Google Scholar]
- 10. Strippoli MP, Frischer T, Barbato A, et al. Management of primary ciliary dyskinesia in European children: recommendations and clinical practice. Eur Respir J 2012; 39: 1482–1491. [DOI] [PubMed] [Google Scholar]
- 11. United Nations Statistics Division. Composition of Macro Geographical (Continental) Regions, Geographical Sub-regions and Selected Economic and Other Groupings. United Nations Statistics Division, https://unstats.un.org/unsd/methodology/m49/ (2007, accessed 05 September 2017). [Google Scholar]
- 12. Elborn JS, Hodson M, Bertram C. Implementation of European standards of care for cystic fibrosis—control and treatment of infection. J Cyst Fibros 2008; 8: 211–217. [DOI] [PubMed] [Google Scholar]
- 13. Lee TW, Brownlee KG, Conway SP, et al. Evaluation of a new definition for chronic Pseudomonas aeruginosa infection in cystic fibrosis patients. J Cyst Fibros 2003; 2: 29–34. [DOI] [PubMed] [Google Scholar]
- 14. Cohen-Cymberknoh M, Simanovsky N, Hiller N, et al. Differences in disease expression between primary ciliary dyskinesia and cystic fibrosis with and without pancreatic insufficiency. Chest 2014; 145: 738–744. [DOI] [PubMed] [Google Scholar]
- 15. Rogers GB, Carroll MP, Zain NMM, et al. Complexity, temporal stability, and clinical correlates of airway bacterial community composition in primary ciliary dyskinesia. J Clin Microbiol 2013; 51: 4029–4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cohen-Cymberknoh M, Weigert N, Gileles-Hillel A, et al. Clinical impact of Pseudomonas aeruginosa colonization in patients with primary ciliary dyskinesia. Respir Med 2017; 131: 241–246. [DOI] [PubMed] [Google Scholar]
- 17. Valerius NH, Koch C, Høiby N. Prevention of chronic Pseudomonas aeruginosa colonization in cystic fibrosis by early treatment. Lancet 1991; 21: 725–726. [DOI] [PubMed] [Google Scholar]
- 18. White L, Mirrani G, Grover M, et al. Outcomes of Pseudomonas eradication therapy in patients with non-cystic fibrosis bronchiectasis. Respir Med 2012; 106: 356–360. [DOI] [PubMed] [Google Scholar]
- 19. Frederiksen B, Koch C, Høiby N. Antibiotic treatment of initial colonization with Pseudomonas aeruginosa postpones chronic infection and prevents deterioration of pulmonary function in cystic fibrosis. Pediatr Pulmonol 1997; 23: 330–335. [DOI] [PubMed] [Google Scholar]
- 20. Equi A, Pike SE, Davies J, et al. Use of cough swabs in a cystic fibrosis clinic. Arch Dis Child 2001; 85: 438–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hanshew A, Jetté ME, Tadayon S, et al. A comparison of sampling methods for examining the laryngeal microbiome. PLoS One 2017; 12: e0174765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alanin MC, Johansen HK, Aanes K, et al. Simultaneous sinus and lung infections in patients with primary ciliary dyskinesia. Acta Otolaryngol 2015: 135: 58–63. [DOI] [PubMed] [Google Scholar]
- 23. Pressler T, Karpati F, Granström M, et al. Diagnostic significance of measurements of specific IgG antibodies to Pseudomonas aeruginosa by three different serological methods. J Cyst Fibros 2009; 8: 37–42. [DOI] [PubMed] [Google Scholar]
- 24. Cohen-Cymberknoh M, Gilead N, Gartner S, et al. Eradication failure of newly acquired Pseudomonas aeruginosa isolates in cystic fibrosis. J Cyst Fibros 2016; 15: 776–782. [DOI] [PubMed] [Google Scholar]
- 25. Kuehni CE, Frischer T, Strippoli MP, et al. Factors influencing age at diagnosis of primary ciliary dyskinesia in European children. Eur Respir J 2010; 36: 1248–1258. [DOI] [PubMed] [Google Scholar]
- 26. Moriarty TF, McElnay JC, Elborn JS, et al. Sputum antibiotic concentrations: implications of cystic fibrosis lung infection. Pediatr Pulmonol 2007; 42: 1008–1017. [DOI] [PubMed] [Google Scholar]
- 27. Cheng K, Smyth AL, Govan JR, et al. Spread of β-lactam-resistant Pseudomonas aeruginosa in a cystic fibrosis clinic. Lancet 1996; 348: 639–642. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, On_line_supplement_resubmission_CRD for Variation in treatment strategies for the eradication of Pseudomonas aeruginosa in primary ciliary dyskinesia across European centers by Suzanne Crowley, Mathias Geldermann Holgersen, and Kim Gjerum Nielsen in Chronic Respiratory Disease