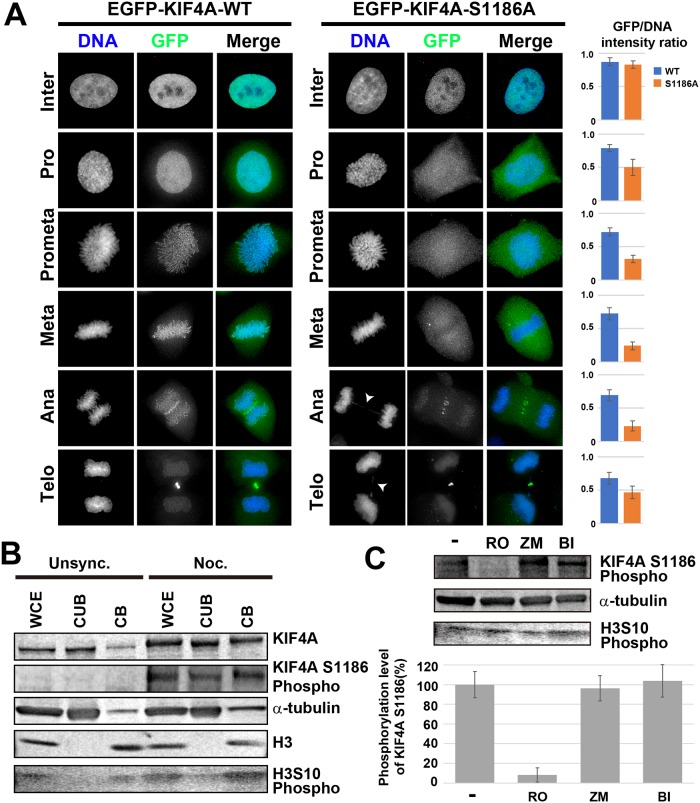
Fig 2. Cdk1-dependent phosphorylation of KIF4A at S1186 regulates the chromosomal localization of KIF4A during mitosis.
(A) Localization of EGFP-KIF4A WT or S1186A was analyzed in fixed cells immunostained with anti-GFP (green) antibody. DNA was counterstained with Hoechst 33342 (blue). Arrowheads indicate chromosome bridge formation. Bar, 5 μm. EGFP-KIF4A WT localized to the chromosome, whereas the localization of EGFP-KIF4A S1186A to the chromosomes was severely decreased after NEB. Bar graphs show GFP/DNA intensity ratios in each mitotic stage (n = 50). (B) Unsynchronous and nocodazole-treated HeLa cells were fractionated into the chromatin-unbound fraction (CUB) and chromatin-binding fraction (CB), respectively, and whole cell extracts (WCE) were also analyzed. The phosphorylation of KIF4A at S1186 increased in mitotic cells, particularly in the chromatin-binding fraction. Alpha-tubulin and histone H3 were also detected as cytoplasmic protein and chromatin protein controls, respectively. Phosphorylation of histone H3 at S10 was detected to confirm the increase in mitotic cells. (C) Mitotic cells were treated with 10 μM RO-3306 (RO), 4 μM ZM447439 (ZM), or 100 nM BI2536 (BI) for 2 h. Cells were then corrected and whole cell extracts were analyzed by WB. Alpha-tubulin was detected as a loading control. KIF4A phosphorylation at S1186 was reduced by RO treatment, indicating that KIF4A S1186 was phosphorylated by Cdk1.H3S10 phosphorylation was detected as mitotic marker, but the intensity was decreased by ZM treatment, as it is phosphorylated by aurora kinase B. The band intensity of phosphorylated KIF4A S1186 was quantified (n = 3).