Abstract
Objective
This study aimed to examine the association between age at menarche and a range of cardiovascular disease (CVD) risk factors at 17 and 20 years of age, and whether this was influenced by childhood body mass index (BMI).
Methods
Of the 1413 girls born in the Western Australian Pregnancy Cohort (Raine) Study, 846 had age at menarche recorded. Subsequently 557 underwent metabolic assessment at 17 years and 541 at 20 years. Associations between age at menarche and cardiovascular risk factors, and being in a high-risk metabolic cluster at 17 and 20 years, or having the metabolic syndrome at 20 years, were investigated by linear mixed effects and logistic regressions, respectively.
Results
Each year later of onset of menarche was associated with a 0.75 kg/m2 reduction in BMI (coefficient -0.75 [95%CI -1.06, -0.44]), and an approximate 30% reduction in the odds of being in the high-risk metabolic cluster at 17 years (OR = 0.73 [95%CI 0.57, 0.94]) and 20 years of age (OR = 0.68 [95%CI 0.52, 0.87]), and a 40% reduction in the odds of having the metabolic syndrome at 20 years (OR = 0.60 [95% CI 0.41, 0.88]). These data show earlier age at menarche was associated with increased BMI and odds of being in the high-risk metabolic cluster at 17 and 20 years, and increased odds of having the metabolic syndrome at 20 years. However, these associations were no longer statistically significant after adjustment for BMI at age 8 years. Current smoking, alcohol consumption, physical activity, socio-economic status, or hormonal contraceptives use did not affect these associations.
Conclusions
Earlier age at menarche may be indicative of a higher risk profile for CVD in young adulthood. Our findings suggest that targeted interventions to reduce BMI in girls who experience menarche at younger age may reduce CVD risk in the future.
Introduction
Cardiovascular disease (CVD) is the leading cause of mortality in women in Western countries [1], and it has been suggested that the origins of CVD may initiate at an early age. Hence identification and intervention for these early life modifiable factors to prevent CVD in later life is essential [2]. The American Heart Association state [3]: “The primary focus is on adult cardiovascular health and disease prevention, but critical to achievement of this goal is maintenance of ideal cardiovascular health from birth through childhood to young adulthood and beyond.” Studies have shown that atherosclerotic disease is present at an early age. For example, over 70% of American soldiers killed in the Korean war, with a mean age of 22 years, had documented presence of atherosclerosis at post-mortem examination [4]. Hence identification of childhood cardiovascular risk factors that may predict adult cardiometabolic risk is essential.
Earlier onset of puberty and age at menarche have been linked to a broad range of adult chronic diseases [5], including cardio-metabolic health in adulthood [6, 7]. Earlier age at menarche has been associated with increased levels of risk factors for CVD including obesity and hypertension [8], left ventricular dysfunction [9], type 2 diabetes [10], and the metabolic syndrome [11] in adulthood. Moreover, there is evidence for a relation between menarche and morbidity from CVD, with results from meta-analysis suggesting an association between early menarche and higher CVD related risk [12]. However, most published studies have relied on retrospective recall of age at menarche, sometimes over many decades, which is not considered reliable [13], and have not adjusted for key drivers of menarche such as childhood BMI or lifestyle factors such as smoking which likely modify this association [12]. Despite extensive published studies of the timing of age at menarche and its relationship with greater risk of CVD [7], there is little understanding of the mechanisms involved. Greater childhood BMI is a predictor of earlier age at menarche [14], and is associated with increased CVD risk if it persists into adulthood. However it is not clear whether earlier age at menarche is an independent risk factor for CVD in adulthood or whether greater childhood BMI is a major confounder in the relationship between age at menarche and increased CVD risk later in life [15]. These issues are of increasing importance, given the global increase in childhood overweight and obesity.
We have previously demonstrated that in girls from the Western Australian Pregnancy Cohort (Raine) Study, being born with a birthweight below expected by gestation at delivery with adjustment for mother’s height, age and parity, and subsequently having a body mass index (BMI) above average at 8 years of age, was a significant predictor of early age at menarche [16]. Consequently within this same contemporary cohort of girls, we aimed to investigate the association between age at menarche and a range of CVD risk factors at 17 and 20 years of age, and whether this was influenced by childhood BMI.
Methods
Study population
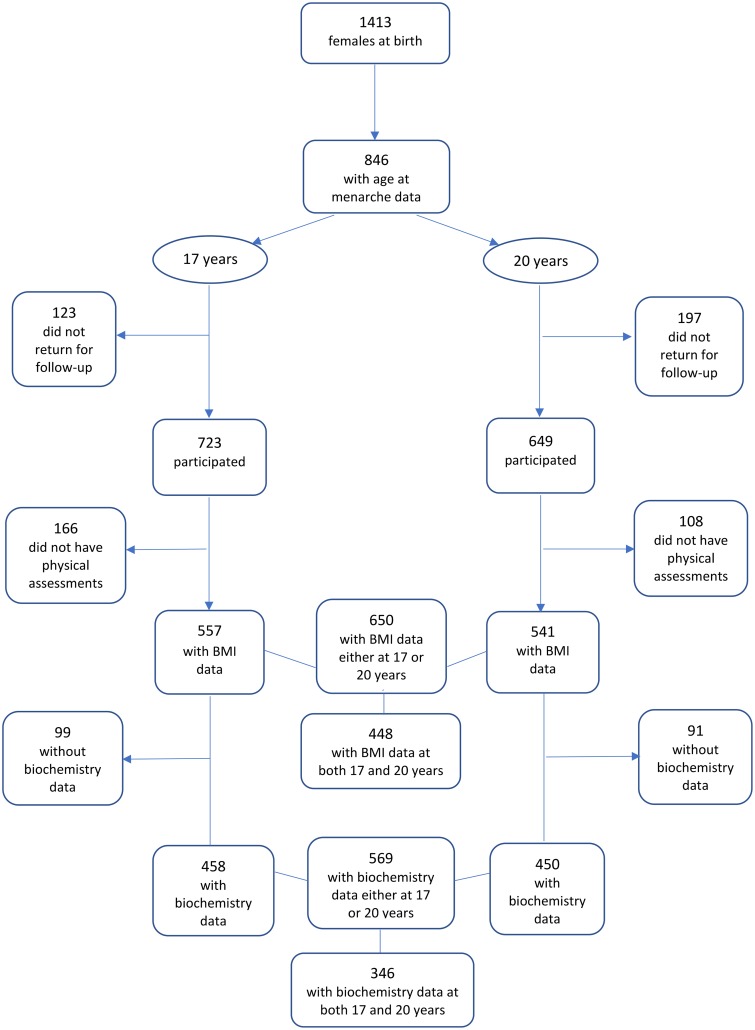
The Western Australian Pregnancy Cohort (Raine) Study, is an on-going longitudinal population-based cohort study in which 2868 live births from 2900 women recruited from King Edward Memorial Hospital and nearby clinics in Perth, Western Australia, were enrolled at 18 weeks of pregnancy between 1989 and 1991. The study population was comprised of 85% Caucasian women. Details of the study are published elsewhere [17]. The study was approved by the Human Ethics Committees of King Edward Memorial Hospital and Princess Margaret Hospital for Children in Perth. Written informed consent was obtained from the mother/primary care giver and the participants at 17 and 20 years of age. The current analysis was based on data from girls participating in assessments at 8, 10, 14, 17 and 20 years of age, and information obtained from the mothers during pregnancy. Fig 1 provides a flow diagram of the study samples. As in most longitudinal studies, loss to follow-up from the original population is inevitable. However, the Raine Study participants remain representative of the Western Australian population, and participants and non-participants at each cohort review remain constant across a number of socio-anthropological and clinical characteristics [18].
Fig 1. Flow diagram of females attending the Raine Study 17-year and 20-year reviews.
Age at menarche
Information on age at menarche calculated to the nearest month was obtained using a purpose-designed questionnaire [19] at 8, 10, 14 and 17 years of age. Mothers or caregivers were asked whether their daughter had experienced menarche since the last study follow-up examination, and to report the exact date of the onset of the first and subsequent 3 menstrual periods. Menarche was defined as the date of the onset of the first menstrual period [20].
Anthropometric and clinical data
At 17 and 20 years, fasting venous blood samples were obtained for the assessment of glucose, insulin, total cholesterol, triglycerides, high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C). Homeostasis model of assessment for insulin resistance (HOMA-IR), an estimate of insulin resistance was calculated as insulin (mU/L) x glucose (mmol/L) / 22.5. After resting for 5 minutes, supine systolic blood pressure (SBP) and diastolic blood pressure (DBP) were recorded using an oscillometric sphygmomanometer (Dinamap Pro Care 100; Soma Technology, Bloomfield, Connecticut, USA), and the last 5 of 6 readings were averaged. Wearing minimal clothing and without shoes, participants were weighted with a Wedderburn Chair Scale (nearest 100 g), and height measured with a Holtain Stadiometer (nearest 0.1 cm). The metabolic syndrome was defined according to the criteria of the International Diabetes Federation [21]: waist circumference ⩾ 90th percentile and at least two of the following factors present: (a) triglycerides ⩾ 1.7 mM; (b) HDL-C < 1.03 mM; (c) SBP ≥ 130 mmHg or DBP ≥ 85 mmHg; and (d) fasting plasma glucose ≥ 5.6 mM.
Hormonal contraceptive (HC) use information was obtained from the question, ‘In the last 6 months, have you taken any prescription medication(s) e.g. the Pill?’ (if yes, ‘which medication(s), and are you still taking it?’). At year 20, HC use included any forms of HC including the progestogen-releasing intrauterine device and subdermal implants.
Maternal pre-pregnancy weight was obtained from self-report, and height was measured at 18 weeks of gestation.
Socio-behavioural data
Socio-behavioural features at 17 and 20 years were assessed via a computer-based questionnaire. An alcohol drinker was defined as consuming alcohol at any level in a day during the last 7 days. The number of cigarettes consumed each day in the last 7 days was recorded, from the questions ‘Have you ever smoked cigarettes in the past 12 months?” and “Have you smoked cigarettes in the past 4 weeks?’ Maternal smoking in pregnancy was self-reported by the mother from the question ‘Do you smoke now?’ with the number of cigarettes consumed. Physical activity at year 17 was assessed from the question, ‘How many hours do you usually exercise in your free time in a week, so much that you get out of breath or sweat?’, from which a dichotomous variable with the cut-point at ≥ 4 hours per week was created. Socioeconomic status was assessed from annual family income (Australian dollars) at 17 years.
In our investigations, a priori variables included maternal smoking in pregnancy, maternal pre-pregnancy BMI, birth weight, BMI at 8 years, physical activity and family income at 17 years, and cigarette smoking, alcohol consumption, and HC use at 17 and 20 years. These factors have been identified as being potentially associated with cardiovascular outcomes.
Statistical analysis
Age at menarche was analysed as a continuous variable; however, for the purpose of describing the sample characteristics, a 3-level categorical variable for age at menarche (≤ 11y, 12-13y, and ≥ 14y) was constructed. CVD risk factors were all analysed as continuous variables. A binary metabolic cluster outcome identifying high and low metabolic risk (separately for 17 yr and 20 yr) was also investigated. This cluster variable was derived from triglycerides, BMI, HOMA-IR, and SBP, using a two-step cluster analysis [22], involving a scalable cluster algorithm. The linearity of the relationship between age at menarche and a continuous outcome was assessed with multivariable spline modelling.
Hierarchical linear and logistic mixed effects regression models were employed to assess the differences between 17 and 20 years for continuous and categorical CVD variables (with the xtmixed and xtmelogit procedures in Stata, respectively). These models utilise maximum likelihood estimation (MLE) which retains all available data including participants with only a single follow up assessment in the analysis. This is known to result in unbiased estimates if missing data are missing at random. Linear mixed models with MLE were also employed to examine the relation between age at menarche and the development of CVD risk factors from years 17 to 20. Time was treated as a 2-level factor variable. Hierarchical models were required to adjust for potential correlation between a small number of siblings present in the sample. Because insulin data were left censored, Tobit regression (the xttobit procedure) was used for analysis. The xttobit command does not allow an adjustment for correlated siblings data, however bootstrapping was employed to obtain robust standard errors.
Models for each CVD risk factor initially included age at menarche and time, to account for the change of the risk factor between 17 and 20 years of age. In models with significant associations with age at menarche, BMI at age 8 years was then included to examine the effect of childhood adiposity on the relationship. The remaining a priori covariates were then added and a manual backward stepwise elimination process employed, in which non-significant covariates in the model were removed, with specific focus on any change in the coefficient for age at menarche that signified confounding, and p-values at each step. Retained in the final multivariable models were all factors still significantly associated with the outcome or influential in the model. Further, to investigate the influence of a priori covariates on this relationship, the interaction between age at menarche and each covariate was tested. The Holm’s step-down procedure was used to identify a significant association after adjusting for multiple comparisons.
Given the sample specific derivation of the metabolic cluster variable at each time point, cross-sectional logistic regression analyses were performed for each year, along with the metabolic syndrome at year 20, to investigate the associations with age at menarche. A per family cluster variance adjustment was incorporated to account for potential correlation between siblings. The model building approach used for continuous outcomes was also adopted for the metabolic outcomes. Due to the small number of participants having the metabolic syndrome, the final model for this variable was bootstrapped to obtain p values that were robust to overfitting.
All analyses were performed using Stata version 13 (StataCorp, College Station, Texas, USA). Results are interpreted with statistical significance set at p < 0.05.
Results
The phenotypic, biochemical and socio-behavioural characteristics of 650 girls, who had complete data on age at menarche and BMI either at 17 or 20 years (n = 557 and n = 541, respectively) are presented in Table 1. Among these participants, 448 had data at both 17 and 20 years. The average age at menarche was 12.7 years (95% CI 12.6, 12.8). Participants’ BMI, SBP, DBP, cholesterol, LDL-C and glucose levels increased over the 17 to 20 year period. Insulin levels and HOMA-IR were significantly higher at age 17 years compare to age 20 years. These data corroborate with recent observations of an increase in insulin resistance in adolescents during the pubertal period [23]. At 20 years of age, 60% were HC users, 68% consumed alcohol, and 12% smoked cigarettes. The prevalence of smoking declined significantly over the period of 17 to 20 years. Approximately 22% of the participants’ mothers smoked in pregnancy. Descriptive characteristics of the study participants at years 17 and 20 according to a 3-level age at menarche (≤ 11y, 12-13y, and ≥ 14y) are demonstrated in Table 2.
Table 1. Characteristics of the study participants (N = 650).
| Early life to menarche | Year 17 (n = 557) |
Year 20 (n = 541) |
P value | |
|---|---|---|---|---|
| Maternal smoking in pregnancy % | 22.5 | |||
| Maternal pre-pregnancy BMI, kg/m2 | 22.4 (4.3) | |||
| Age at menarche, yr | 12.7 (1.1) | |||
| BMI at year 8, kg/m2 | 16.8 (2.5) | |||
| BMI in early adulthood, kg/m2 | 23.1 (4.5) | 24.3 (5.4) | <0.001 | |
| SBP, mmHg | 108.8 (9) | 111.1 (9.9) | <0.001 | |
| DBP, mmHg | 59.4 (6.4) | 65.4 (7.2) | <0.001 | |
| Total cholesterol, mmol/L | 4.3 (0.7) | 4.5 (0.7) | <0.001 | |
| Triglycerides, mmol/L | 1.0 (0.5) | 1.1 (0.5) | 0.228 | |
| HDL-C, mmol/L | 1.4 (0.3) | 1.5 (0.3) | <0.001 | |
| LDL-C, mmol/L | 2.4 (0.6) | 2.6 (0.6) | <0.001 | |
| Glucose, mmol/L | 4.6 (0.4) | 4.9 (0.7) | <0.001 | |
| Insulin, mU/L † | 8.0 (5.4, 11.4) | 2.8 (1.9, 6.0) | <0.001 | |
| HOMA-IR † | 1.6 (1.1, 2.4) | 0.6 (0.4, 1.3) | <0.001 | |
| Smoker *, n % | 124 (22.3) | 62 (12) | <0.001 | |
| Alcohol drinker ǂ, n % | 281 (50.5) | 445 (68.4) | <0.001 | |
| HC user, n % | 175 (30.3) | 335 (60.3) | <0.001 | |
| High-risk metabolic cluster, n % | 87 (19.1) | 96 (21.5) | N/A¶ | |
| Metabolic Syndrome, n% | 24 (4.3) | |||
| High physical activity §, n % | 105 (21) | |||
| Annual family income at year 17, n % | ||||
| ≤A$35 000 | 69 (12.9) | |||
| A$35 001 to ≤ A$78 000 | 177 (33.1) | |||
| >A$78 000 | 289 (54) |
Data are expressed as mean (standard deviation), median (Q1, Q3) †, or n (percentage).
Abbreviations: BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; HOMA-IR, homeostasis model of assessment for insulin resistance; HC, hormonal contraceptives.
* Smoking ≥ 1 cigarette in a week.
ǂ Consuming alcohol at any level over the last 7 days.
§ Having ≥ 4 hr of exercise in free time per week.
¶ Different criteria were used for years 17 and 20, respectively.
Table 2. CVD risk factors of the study participants by categories of age at menarche (N = 650).
| Year 17 (n = 557) |
Year 20 (n = 541) |
|||||
|---|---|---|---|---|---|---|
| Categories of age at menarche |
≤ 11y (n = 124) |
12-13y (n = 358) |
≥ 14y (n = 75) |
≤ 11y (n = 118) |
12-13y (n = 351) |
≥ 14y (n = 72) |
| BMI at year 8, kg/m2 | 17.7 (3.2) | 16.6 (2.2) | 16.2 (2.4) | 17.4 (2.9) | 16.6 (2.2) | 15.9 (1.9) |
| BMI in early adulthood, kg/m2 | 24.2 (5.3) | 22.8 (4) | 22.7 (5.3) | 25.4 (5.9) | 24.1 (5) | 23.8 (5.9) |
| Waist circumference, cm † | 76.1 (71, 8) | 75 (69.6, 82.3) | 75.3 (70.3, 82.9) | 75.6 (69.8, 82.8) | 73.9 (68, 81.8) | 74.4 (68.7, 81.5) |
| SBP, mmHg | 109.1 (8.9) | 108.9 (8.9) | 107.9 (9.4) | 112.1 (10.3) | 110.9 (10.1) | 110.3 (8.8) |
| DBP, mmHg | 59.7 (6.3) | 59.7 (6.5) | 57.8 (6.1) | 65.6 (6.6) | 65.4 (7.4) | 64.8 (6.7) |
| Total cholesterol, mmol/L | 4.4 (0.7) | 4.3 (0.7) | 4.23 (0.7) | 4.6 (0.7) | 4.5 (0.8) | 4.6 (0.8) |
| Triglycerides, mmol/L | 1.0 (0.4) | 1.0 (0.5) | 1.0 (0.5) | 1.0 (0.5) | 1.1 (0.4) | 1.1 (0.5) |
| HDL-C, mmol/L | 1.4 (0.3) | 1.4 (0.3) | 1.4 (0.3) | 1.5 (0.3) | 1.5 (0.3) | 1.5 (0.3) |
| LDL-C, mmol/L | 2.5 (0.6) | 2.4 (0.7) | 2.4 (0.6) | 2.6 (0.6) | 2.5 (0.6) | 2.6 (0.6) |
| Glucose, mmol/L | 4.6 (0.4) | 4.6 (0.4) | 4.7 (0.4) | 4.9 (0.4) | 4.9 (0.8) | 4.8 (0.3) |
| Insulin, mU/L † | 8.3 (5.3, 12.3) | 7.9 (5.42, 10.9) | 8.3 (5.9, 11.5) | 3.2 (1.9, 6.0) | 2.9 (1.9, 6.0) | 2.6 (1.9, 6.1) |
| HOMA-IR † | 1.7 (1.0, 2.5) | 1.6 (1.1, 2.3) | 1.6 (1.3, 2.5) | 0.6 (0.4, 1.3) | 0.6 (0.4, 1.4) | 0.6 (0.4, 1.3) |
| High-risk metabolic cluster, n (%) | 27 (26.7) | 46 (15.9) | 14 (21.2) | 30 (30.6) | 55 (19.2) | 11 (17.7) |
| Metabolic Syndrome, n (%) | 9 (7.4) | 12 (3.3) | 3 (4.2) | |||
| Smoker *, n (%) | 29 (23.5) | 82 (22.8) | 13 (17.3) | 12 (11) | 42 (12.5) | 8 (11.4) |
| Alcohol drinker ǂ, n (%) | 58 (46.8) | 190 (53.2) | 33 (44) | 92 (63) | 293 (69.7) | 60 (71.4) |
| Physical activity§, ≥4 hr/wk n (%) | 22 (19.8) | 65 (20.1) | 18 (27.7) | |||
| HC user, n (%) | 40 (31.2) | 113 (30.2) | 22 (29.3) | 65 (53.3) | 226 (62.8) | 44 (60.3) |
| Family Income, n (%) | ||||||
| ≤A$35 000 | 14 (12.7) | 41 (13.1) | 10 (15.9) | |||
| A$35 001 to ≤ A$78 000 | 43 (39.1) | 100 (32) | 22 (34.9) | |||
| >A$78 000 | 53 (48.2) | 172 (55) | 31 (49.2) | |||
Data are expressed as mean (standard deviation), median (Q1, Q3) †, or n (percentage).
Abbreviations: CVD, cardiovascular disease; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; HOMA-IR, homeostasis model of assessment for insulin resistance; HC, hormonal contraceptives.
* Smoking ≥ 1 cigarette in a week;
ǂ Consuming alcohol at any level over the last 7 days;
§ Having ≥ 4 hr of exercise in free time per week.
There was a linear relationship between age at menarche and current BMI with no evidence that the association varied between 17 and 20 years. Therefore, a single estimate of the effect is reported for both time periods.
After adjusting for the change in the CVD outcome over time and for potential confounders, there was a significant inverse association between age at menarche and BMI at age 17 and 20 years (Table 3). Each later year in age at menarche was associated with a 0.75 kg/m2 reduction in BMI (coefficient -0.75 [95%CI -1.06, -0.44]). This association shows increasing BMI with earlier age at menarche. However, this association was no longer statistically significant after further adjustment for BMI at age 8 years (coefficient -0.16 [95%CI -0.42, 0.08]). There was no significant interaction of age at menarche and BMI (expressed as a continuous variable [p = 0.209] (S1 Table) or as tertiles [p = 0.585] (S2 Table)) at age 8 years. There was no significant influence of current smoking, alcohol consumption, physical activity, socio-economic status, or HC use, on the association between age at menarche and BMI at 17 and 20 years. Further, after multiple testing correction, there was no significant association between age at menarche and other CVD risk factors, including SBP, DBP, total cholesterol, HDL-C, LDL-C, triglycerides, glucose, insulin, and HOMA-IR, after adjustment for the change over time in the CVD risk factors (S3 Table).
Table 3. Longitudinal hierarchical linear mixed models of the association between age at menarche and BMI at years 17 to 20.
| Models | N | Regression coefficient for age at menarche | 95% C I | P value |
|---|---|---|---|---|
| Included Time ¶ | 584 | -0.82 | -1.16, -0.47 | <0.001 |
| Included Time ¶ + significant Covariates ǂ * | 584 | -0.75 | -1.06, -0.44 | <0.001 |
| Included Time ¶ + Covariates ǂ + BMI at 8 years of age * | 584 | -0.16 | -0.42, 0.08 | 0.188 |
Abbreviations: CI, confidence interval; BMI, body mass index
¶ Representing the change in the outcome between ages 17 and 20 years
ǂ Including alcohol consumption and maternal pre-pregnancy BMI
* Variables explored for significant associations include: maternal pre-pregnancy BMI, maternal smoking in pregnancy, birth weight, current smoking, alcohol consumption, hormonal contraceptive use, physical activity, and family income
At 17 and 20 years of age, 19.1% and 21.5%, respectively, were classified as being in a high-risk metabolic cluster, and 4.3% with the metabolic syndrome (Table 1). Characteristics of the high- and low-risk metabolic clusters at 17 and 20 years, and the metabolic syndrome at year 20, are shown in S4 Table. Models for the relationship between age at menarche and the metabolic clusters at years 17 and 20, respectively, and the metabolic syndrome at year 20, are shown in Table 4. For each year of later onset in age at menarche, there was an approximate 30% reduction in the odds of being in the high-risk metabolic cluster at 17 years (OR = 0.73 [95%CI 0.57, 0.94]) and 20 years (OR = 0.68 [95%CI 0.52, 0.87]), and a 40% reduction in the odds of having the metabolic syndrome at year 20 (OR = 0.60 [95% CI 0.41, 0.88]). These data show earlier age at menarche was associated with increased odds of being in the high-risk metabolic cluster or of having the metabolic syndrome. However, these associations were not significant after adjustment for BMI at 8 years. There were no interactions between significant covariates and age at menarche (S5 Table) in the metabolic cluster models at age 17 or 20 years, or the metabolic syndrome at year 20.
Table 4. Logistic regression models of the association of age at menarche with the metabolic clusters at years 17 and 20 and the metabolic syndrome at year 20.
| Metabolic clusters | Metabolic syndrome | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Models | Year 17 | Year 20 | Year 20 | |||||||||
| N | OR | 95% CI | P value | N | OR | 95% CI | P value | N | OR | 95% CI | P value | |
| Univariate | 417 | 0.69 | 0.54, 0.88 | 0.003 | 404 | 0.62 | 0.49, 0.80 | <0.001 | 500 | 0.58 | 0.40, 0.84 | 0.004 |
| Included covariates ¶ * | 417 | 0.73 | 0.57, 0.94 | 0.0015 | 404 | 0.68 | 0.52, 0.87 | 0.003 | 500 | 0.60 | 0.41, 0.88 | 0.009 |
|
Included covariates
¶ + BMI at 8 years of age * |
417 | 0.89 | 0.68, 1.16 | 0.392 | 404 | 0.80 | 0.61, 1.05 | 0.114 | 500 | 0.73 | 0.47, 1.11 | 0.145 |
Abbreviations: OR, odds ratio; CI, confidence interval; BMI, body mass index
¶ Including maternal smoking in pregnancy and maternal pre-pregnancy BMI (y 17), and maternal pre-pregnancy BMI (y 20)
* Variables explored for significant associations include: maternal pre-pregnancy BMI, maternal smoking in pregnancy, birth weight, current smoking, alcohol consumption, hormonal contraceptive use, physical activity, and family income
Discussion
In a large well-phenotyped pregnancy cohort with prospectively measured age at menarche, we have shown that earlier age at menarche was associated with higher BMI levels at age 17 and 20 years, and increased odds of being in a cluster of high metabolic risk at both ages or of having the metabolic syndrome at 20 years. Importantly, our findings show these associations were largely accounted for by elevated BMI in childhood. There was no significant association between age at menarche and a range of CVD risk factors at 17 and 20 years, including blood pressure, fasting lipids, glucose, insulin and HOMA-IR. Our findings suggest that early age at menarche is not itself an independent risk factor for increased cardio-metabolic risk in late adolescence and young adulthood. Earlier age at menarche (primary school age) may be indicative of a higher risk profile for CVD in young adulthood and hence be a marker for girls requiring earlier intervention (such as weight control) to modify this risk.
Early age at menarche may be viewed as a sentinel event; hence studies of health intervention strategies to mitigate against the subsequent development of cardiometabolic disorders are essential. Studies have shown that the age at menarche has been declining over time, and related to increased adiposity in girls [24]. Over the last two centuries in Europe the mean age at menarche has decreased by 44 days for every five year birth cohort, being greatest in Spain and Germany [25]. Numerous physiological, social and inherited factors are thought to contribute to age at menarche including pre-menarcheal endocrine and nutritional status, and body adiposity [26]. There is extensive evidence that the onset and tempo of puberty are influenced by childhood adiposity [27]. We have previously reported from the Raine cohort that being born with a birthweight below expected by gestation at delivery with adjustment for mother’s height, age and parity, and subsequently having a BMI above average at 8 years of age, significantly predicted early age of menarche [16]. Our current findings suggest that age at menarche is driven by BMI in childhood, and subsequent CVD risk profiles in young adulthood are associated with childhood BMI and not independently with age at menarche.
We focused on BMI related outcomes in this young adulthood period, as a meta-analysis previously reported that the effects of age at menarche on adulthood BMI were greater in younger women [12]. Several studies have reported a significant association between earlier age at menarche and higher adiposity levels in adulthood [8, 12], however only a few assessed the confounding role of childhood BMI [28, 29]. An independent effect of age at menarche on later adiposity was reported in studies with childhood BMI measured at 4 to 6 years [28], but not in others [29], including the present study in which BMI was assessed at 8 years. Our study had the advantage of prospective measures of BMI at 8 years of age, closer to the onset of pubertal maturation and menarche.
The strength of our study has been the use of a large well-characterised contemporary population-based pregnancy cohort, with comprehensive anthropometric, clinical and socio-behavioural data prospectively collected through serial surveys from pregnancy throughout childhood and adolescence to early adulthood. Age at menarche was calculated to the nearest month using a purpose-designed questionnaire at 8, 10, 14 and 17 years of age. Cohort effects have been an important methodological issue in assessing the impact of age at menarche on height [25] or adult cardiovascular disease [30]. By using a single birth cohort, we have excluded cohort bias. Furthermore, we employed hierarchical mixed effects statistical analysis that enabled a longitudinal assessment of the development of CVD outcomes over time, in models that considered the confounding effects of a range of covariates and their interaction with time. Our population was predominantly Caucasian, and was largely of middle to upper level socio economic status (SES). This limits the ability of our study to comment on how ethnicity may impact on the relationship between age at menarche and CVD risk, but also reduces important sources of bias since both ethnicity and SES are known to influence age at menarche [31].
In summary, our study shows that age at menarche is not an independent predictor for cardiometabolic risk in late adolescence and young adulthood. Our findings suggest that the association between early menarche and cardiometabolic risk factors is due to underlying childhood adiposity, suggesting that targeting childhood adiposity may increase both age at menarche and CV health in later life. For girls who experience menarche at a younger (primary school) age who have modifiable risk factors for CVD (such as obesity) targeted interventions such as weight reduction may reduce the risk of CVD in later life.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability
The ethical consent for the Western Australian Pregnancy Cohort (Raine) Study data does not allow the data to be placed in the public domain. However the data are available for bona fide research as well as, if required, auditing of published findings. Details of the approval process to follow to access the data are provided at the Raine Study website (www.rainestudy.org.au). We confirm the authors had no special access privileges.
Funding Statement
This work was supported by Core funding of the Raine Study is contributed by the University of Western Australia (UWA), the Telethon Kids Institute, the Raine Medical Research Foundation, the UWA Faculty of Medicine, Dentistry and Health Science, the Women and Infants Research Foundation, Curtin University, Edith Cowan University, Murdoch University, and the University of Notre Dame Australia. The Raine Study has long-term support from the National Health and Medical Research Council of Australia. The data collection of the 17 year follow-up of the Raine Study was funded by an NHMRC grant (Beilin et al, ID 403981). Rae-Chi Huang and Trevor A Mori are supported by NHMRC fellowships 1053384 and 1042255, respectively. Martha Hickey is supported by NHMRC Practitioner Fellowship APP1058935. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd-Jones DM, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women—2011 update: A guideline from the American Heart Association. J Am Coll Cardiol 2011; 57: 1404–1423. 10.1016/j.jacc.2011.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daniels SR, Pratt CA and Hayman LL. Reduction of risk for cardiovascular disease in children and adolescents. Circulation. 2011;124:1673–86. 10.1161/CIRCULATIONAHA.110.016170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinberger J, Daniels SR, Hagberg N, Isasi C, Kelly AS, Lloyd-Jones D, et al. Cardiovascular Health Promotion in Children: Challenges and Opportunities for 2020 and Beyond: A Scientific Statement From the American Heart Association. Circulation. 2016;134:e236–55. 10.1161/CIR.0000000000000441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Enos WF, Holmes RH and Beyer J. Coronary disease among United States soldiers killed in action in Korea; preliminary report. JAMA. 1953;152:1090–3. [DOI] [PubMed] [Google Scholar]
- 5.Day FR, Elks CE, Murray A, Ong KK and Perry JRB. Puberty timing associated with diabetes, cardiovascular disease and also diverse health outcomes in men and women: the UK Biobank Study. Sci Rep. 2015;5:11208 10.1038/srep11208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lakshman R, Forouhi NG, Sharp SJ, Luben R, Bingham SA, Khaw K-T, et al. Early age at menarche associated with cardiovascular disease and mortality. J Clin Endocrinol Metab. 2009;94: 4953–4960. 10.1210/jc.2009-1789 [DOI] [PubMed] [Google Scholar]
- 7.Canoy D, Beral V, Balkwill A, Wright FL, Kroll ME, Reeves GK, et al. Age at menarche and risks of coronary heart and other vascular diseases in a large UK cohort. Circulation 2015; 131: 237–244. 10.1161/CIRCULATIONAHA.114.010070 [DOI] [PubMed] [Google Scholar]
- 8.Dreyfus J, Jacobs DR Jr, Mueller N, Schreiner PJ, Moran A, Carnethon MR, et al. Age at Menarche and Cardiometabolic Risk in Adulthood: The Coronary Artery Risk Development in Young Adults Study. J Pediatr. 2015;167:344–352.e1. 10.1016/j.jpeds.2015.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryu S, Chang Y, Kang JG, Sung J, Kim J-Y, Jung H-S, et al. Association of age at menarche with left ventricular diastolic dysfunction in middle-aged women. Cir J. 2018; 82: 708–714. [DOI] [PubMed] [Google Scholar]
- 10.Stöckl D, Döring A, Peters A, Thorand B, Heier M, Huth C, et al. Age at menarche is associated with prediabetes and diabetes in women (aged 32–81 years) from the general population: the KORA F4 Study. Diabetologia. 2012;55:681–688. 10.1007/s00125-011-2410-3 [DOI] [PubMed] [Google Scholar]
- 11.Stöckl D, Meisinger C, Peters A, Thorand B, Huth C, Heier M, et al. Age at menarche and its association with the metabolic syndrome and its components: Results from the KORA F4 Study. PLoS ONE. 2011;6:e26076 10.1371/journal.pone.0026076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prentice P and Viner RM. Pubertal timing and adult obesity and cardiometabolic risk in women and men: a systematic review and meta-analysis. Int J Obes. 2013;37:1036–1043. [DOI] [PubMed] [Google Scholar]
- 13.Żarów R and Cichocka BA. A comparative analysis of estimation of age at menarche by various methods in women participating in the Krakow Longitudinal Growth Study, Poland. Am J Hum Biol. 2008;20:146–148. 10.1002/ajhb.20701 [DOI] [PubMed] [Google Scholar]
- 14.Freedman DS, Khan LK, Serdula MK, Dietz WH, Srinivasan SR, Berenson GS. The relation of menarcheal age to obesity in childhood and adulthood: the Bogalusa Heart Study. BMC Pediatrics. 2003;3:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Celermajer DS and Ayer JGJ. Childhood risk factors for adult cardiovascular disease and primary prevention in childhood. Heart. 2006;92:1701–1706. 10.1136/hrt.2005.081760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sloboda DM, Hart R, Doherty DA, Pennell CE, Hickey M. Age at menarche: Influences of prenatal and postnatal growth. J Clin Endocrinol Metab 2007; 92: 46–50. 10.1210/jc.2006-1378 [DOI] [PubMed] [Google Scholar]
- 17.McKnight CM, Newnham JP, Stanley FJ, Mountain JA, Landau LI, Beilin LJ, et al. Birth of a cohort—the first 20 years of the Raine Study. Med J Aust. 2012;197:608–610. [DOI] [PubMed] [Google Scholar]
- 18.Straker L, Mountain J, Jacques A, White S, Smith A, Landau L, et al. Cohort Profile: The Western Australian Pregnancy Cohort (Raine) Study—Generation 2. Int J Epidemiol. 2017;46:1384–5j. 10.1093/ije/dyw308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morris NM and Udry JR. Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adolesc. 1980;9:271–280. 10.1007/BF02088471 [DOI] [PubMed] [Google Scholar]
- 20.Whitehouse AJO, Maybery MT, Hickey M and Sloboda DM. Brief report: Autistic-like traits in childhood predict later age at menarche in girls. J Autism Dev Disord 2011; 41: 1125–1130. 10.1007/s10803-010-1129-1 [DOI] [PubMed] [Google Scholar]
- 21.Alberti KGMM, Zimmet P, Shaw J. Metabolic syndrome—a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23:469–80. 10.1111/j.1464-5491.2006.01858.x [DOI] [PubMed] [Google Scholar]
- 22.Huang RC, Burke V, Newnham JP, Stanley FJ, Kendall GE, Landau LI,et al. Perinatal and childhood origins of cardiovascular disease. Int J Obes. 2006;31:236–244. [DOI] [PubMed] [Google Scholar]
- 23.Hannon TS, Janosky J, Arslanian SA. Longitudinal study of physiologic insulin resistance and metabolic changes of puberty. Pediatr Res. 2006;60:759 10.1203/01.pdr.0000246097.73031.27 [DOI] [PubMed] [Google Scholar]
- 24.Li W, Liu Q, Deng X, Chen Y, Liu S and Story M. Association between obesity and puberty timing: A systematic review and meta-analysis. Int J Environ Res Public Health. 2017;14: 1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Onland-Moret NC, Peeters PHM, van Gils CH, Clavel-Chapelon F, Key T, Tionneland A, et al. Age at menarche in relation to adult height: The EPIC Study. Am J Epidemiol 2005;162:623–632. 10.1093/aje/kwi260 [DOI] [PubMed] [Google Scholar]
- 26.Berkey CS, Gardner JD, Lindsay Frazier A and Colditz GA. Relation of childhood diet and body size to menarche and adolescent growth in girls. Am J Epidemiol 2000;152:446–452. [DOI] [PubMed] [Google Scholar]
- 27.German A, Shmoish M and Hochberg Ze. Predicting pubertal development by infantile and childhood height, BMI, and adiposity rebound. Pediatr Res. 2015;78:445–450. 10.1038/pr.2015.129 [DOI] [PubMed] [Google Scholar]
- 28.Pierce MB and Leon DA. Age at menarche and adult BMI in the Aberdeen Children of the 1950s Cohort Study. Am J Clin Nutr. 2005;82:733–739. 10.1093/ajcn/82.4.733 [DOI] [PubMed] [Google Scholar]
- 29.Kivimäki M, Lawlor DA, Smith GD, Elovainio M, Jokela M, Keltikangas-Järvinen L, et al. Association of age at menarche with cardiovascular risk factors, vascular structure, and function in adulthood: the Cardiovascular Risk in Young Finns Study. Am J Clin Nutr. 2008;87:1876–1882. 10.1093/ajcn/87.6.1876 [DOI] [PubMed] [Google Scholar]
- 30.Yang L, Li L, Millwood IY, Peters SAE, Chen Y, Guo Y, Bian Z, et al. Age at menarche and risk of major cardiovascular diseases: Evidence of birth cohort effects from a prospective study of 300,000 Chinese women. Int J Cardiol. 2017;227:497–502. 10.1016/j.ijcard.2016.10.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karapanou O, Papadimitriou A. Determinants of menarche. Reprod Biol Endocrinol. 2010;8:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
The ethical consent for the Western Australian Pregnancy Cohort (Raine) Study data does not allow the data to be placed in the public domain. However the data are available for bona fide research as well as, if required, auditing of published findings. Details of the approval process to follow to access the data are provided at the Raine Study website (www.rainestudy.org.au). We confirm the authors had no special access privileges.