Abstract
Tumor necrosis factor-like weak inducer of apoptosis (TWEAK), along with its receptor fibroblast growth factor-inducible (Fn)14, is associated with various biological activities including inflammation. However, its role in the pathogenesis of Graves’ orbitopathy (GO) is unknown. In this study, we investigated the mechanism by which TWEAK regulates inflammatory signaling in orbital fibroblasts from GO patients. We found that TWEAK and tumor necrosis factor-α (TNFA) mRNA levels were upregulated in GO as compared to non-GO tissue samples. TWEAK, TNF receptor (TNFR)1, TNFR2, and TNFR superfamily member 12A mRNA, and TWEAK and Fn14 protein levels were increased by interleukin (IL)-1β and TNF-α treatment. Treatment with exogenous recombinant TWEAK increased the transcript and protein expression of the pro-inflammatory cytokines IL-6, IL-8, and monocyte chemoattractant protein-1 to a greater extent in GO than in non-GO cells, while treatment with the anti-Fn14 antibody ITEM4 suppressed TWEAK-induced pro-inflammatory cytokine release and hyaluronan production. Additionally, the serum level of TWEAK was higher in Graves’ disease patients with (341.86 ± 86.3 pg/ml) as compared to those without (294.09 ± 41.44 pg/ml) GO and healthy subjects (255.33 ± 39.38 pg/ml), and was positively correlated with clinical activity score (r = 0.629, P < 0.001) and thyroid binding immunoglobulin level (r = 0.659, P < 0.001). These results demonstrate that TWEAK/Fn14 signaling contributes to GO pathogenesis. Moreover, serum TWEAK level is a potential diagnostic biomarker for inflammatory GO, and modulating TWEAK activity may be an effective therapeutic strategy for suppressing inflammation and tissue remodeling in GO.
Introduction
Graves’ orbitopathy (GO) is an inflammatory autoimmune disorder of the orbit associated with Graves’ disease (GD) that occurs concurrently with thyroid dysfunction in about 40% of cases[1]. An epidemiological study in the United States reported a GO incidence of 16/100,000 in femaile and 2.9/100,000 in male [2], and among 237 GD patients seen at a single center in Europe, 81.9% had no GO at baseline but 2.6% progressed to moderate-to severe GO, and among 237 GD patients seen at a single center in Europe, 81.9% had no GO at baseline but 2.6% progressed to moderate-to-severe GO. Thyroid-stimulating hormone receptor (TSHR) expressed in orbital fibroblasts is an autoimmune target in GO [3–6]. The binding of auto-antibodies to orbital fibroblasts activates T cell-dependent inflammatory processes; activated cluster of differentiation (CD)4+ T cells secrete interleukin (IL)-1, interferon-γ, and tumor necrosis factor (TNF)-α, inducing the expression of TSHR and CD40 on the surface of orbital fibroblasts and promoting the secretion of IL-6, IL-8, fibronectin, type I collagen, and glycosaminoglycans [7–9]. Interaction with CD4+ T cells enhances Graves’ orbital fibroblast activation, proliferation, differentiation, and fat accumulation; expansion of the fibroblast pool is characterized not only by marked infiltration of activated lymphocytes, but also by increased production of hydrophilic hyaluronan in response to cytokines [10,11].
Tumor necrosis factor-like weak inducer of apoptosis (TWEAK) is an apoptosis-inducing ligand belonging to TNF superfamily that modulates various biological processes via its receptor fibroblast growth factor-inducible (Fn)14, which is encoded by TNF receptor superfamily member (TNFRSF)12A. TWEAK regulates inflammation, cell proliferation, angiogenesis, and tissue repair and regeneration [12–14] and induces the production of pro-inflammatory molecules—e.g., matrix metalloproteinase (MMP)1, IL-6, IL-8, monocyte chemotactic protein (MCP)-1, and regulated upon activation normal T cell—that are expressed and secreted by synoviocytes and fibroblasts as well as intercellular adhesion molecule (ICAM)-1, E-selectin, IL-8, and MCP-1 by endothelial cells [14–17]. The pro-inflammatory effects of TWEAK/Fn14 are mediated by several signaling pathways including nuclear factor (NF)-κB, mitogen-activated protein kinase (MAPK), extracellular signal-related kinase (ERK)1/2, c-Jun N-terminal kinase (JNK)1/2, and p38 cascades [18]. The TWEAK/Fn14 interaction is particularly important in synovial inflammation associated with rheumatoid arthritis (RA); higher serum levels of TWEAK, TNF-α, and IL-6 have been reported in RA patients as compared to normal controls [19]. Furthermore, recombinant (r)TWEAK enhanced the production of MCP-1 and macrophage inflammatory protein-2 by synovial cells in collagen-induced arthritis (CIA) mice, whereas a monoclonal antibody (mAb) against TWEAK attenuated synovial proliferation and inflammatory cell accumulation in CIA [20]. Thus, blocking TWEAK signaling suppresses inflammation; in fact, studies of TWEAK and Fn14 knockout mice have shown that this could be an effective treatment strategy for autoimmune diseases such as GO.
The present study investigated the mechanism by which TWEAK/Fn14 signaling contributes to the pathogenesis of inflammation in primary cultures of orbital fibroblasts from GO patients. We found that pro-inflammatory cytokine and hyaluronan production by GO fibroblasts in primary cultures was increased in response to exogenous rTWEAK, an effect that was abrogated by anti-Fn14 mAb. Moreover, serum TWEAK concentration was correlated with clinical activity score (CAS) and TSHR Ab level in GO patients. Our data suggest that TWEAK/Fn14 signaling plays a critical role in the pathogenesis of GO-related inflammation.
Materials and methods
Reagents and chemicals
Recombinant TWEAK, recombinant human IL-1β, and TNF-α were purchased from R&D Systems (Minneapolis, MN, USA). Dulbecco’s Modified Eagle’s Medium (DMEM), fetal bovine serum (FBS), penicillin, and gentamycin were from Hyclone Laboratories (Logan, UT, USA). Anti-Fn14 mAb (ITEM4) was from eBioscience (SanDiego, CA, USA). Inhibitors of MAPK kinase 1 (PD98059), p38 MAPK (SB203580), JNK (SP600125), phosphoinositide 3-kinase (PI3K; LY294002), and NF-κB p65 (SC514) were purchased from Calbiochem (La Jolla, CA, USA).
Subjects and tissue and cell preparation
Orbital tissue specimens were collected from patients during orbital decompression surgery for severe GO (n = 11; eight women and three men, aged 39–55 years). Normal orbital tissue was obtained over the course of orbital surgery for other non-inflammatory diseases from age- and sex-matched control subjects with no history of autoimmune thyroid disease or GO (n = 7; four women and three men, aged 34–53 years). The non-GO subjects underwent orbital wall fracture surgery (n = 3), evisceration (n = 2), and orbital benign mass excision (n = 2). The euthyroid status of GO patients was determined at the time of surgery, and patients had not received steroid or radiation therapy for at least 3 months. Clinical information on GO and non-GO patients is shown in Table 1.
Table 1. Clinical information of patient samples used in an in vitro study.
| Age (years) | sex | CAS | Smoker | Duration of GO (years) | Proptosis R/L (mm) | Surgery performed |
|---|---|---|---|---|---|---|
| GO patients | ||||||
| 46 | M | 3/7 | y | 1.6 | 20/21 | Decompression |
| 39 | F | 2/7 | y | 2.0 | 22/22 | Decompression |
| 40 | M | 0/7 | n | 2.2 | 21/20.5 | Decompression |
| 42 | F | 1/7 | n | 3.0 | 23/23 | Decompression |
| 48 | F | 0/7 | n | 3.2 | 18/18 | Decompression |
| 51 | F | 1/7 | n | 2.6 | 20/20 | Decompression |
| 52 | M | 2/7 | y | 1.5 | 24/24 | Decompression |
| 48 | F | 4/7 | y | 2.1 | 22.5/21 | Decompression |
| 55 | F | 3/7 | n | 1.8 | 22/22 | Decompression |
| 44 | F | 1/7 | n | 2.1 | 20.5/21 | Decompression |
| 42 | F | 0/7 | y | 2.4 | 23/23.5 | Decompression |
| Non-GO control subjects | ||||||
| 43 | M | 0 | y | n/a | n/a | evisceration |
| 44 | F | 0 | y | n/a | n/a | orbital wall fracture |
| 34 | F | 0 | n | n/a | n/a | evisceration |
| 53 | M | 0 | n | n/a | n/a | orbital wall fracture |
| 38 | F | 0 | n | n/a | n/a | orbital wall fracture |
| 47 | M | 0 | n | n/a | n/a | orbital mass excision |
| 42 | F | 0 | n | n/a | n/a | orbital mass excision |
CAS, clinical activity score; GO, Graves’ orbitopathy; n/a, not applicable.
Primary cultures of orbital fibroblasts were established as previously described [21]. Briefly, orbital tissue explants were minced and placed in plastic culture dishes in DMEM:F12 medium with 20% FBS and antibiotics. After orbital adipose tissue had grown out from the explants, monolayers were serially passaged following trypsin/EDTA treatment and cells were incubated in DMEM with 10% FBS and antibiotics. Cells between the second and fifth passages were used for experiments.
Sera for analysis of TWEAK levels were obtained from 56 GO patients, 35 GD patients without GO, and 39 healthy control subjects (S1 Table). All patients were recruited from the Department of Ophthalmology at Severance Hospital, Seoul, Korea, and healthy controls were age and sex-matched healthy control subjects. The institutional review board of Severance Hospital, Yonsei University College of Medicine (Seoul, Korea) approved the study and written, informed consent was obtained from all participants after they had been provided with an explanation of the nature and possible consequences of the study. This study protocol adhered to the tenets of the Declaration of Helsinki.
CAS is based on the classical signs of inflammation and comprises seven items that are scored from 0 to 7. TSHR Ab level was measured using third-generation thyrotropin-binding inhibitory immunoglobulin (TBII) with the automated Cobas electrochemiluminescence immunoassay (Elecsys; Roche Diagnostics GmbH, Penzberg, Germany) according to the manufacturer’s instructions.
Real-time polymerase chain reaction (PCR)
Cells were cultured in 6-cm dishes until they reached confluence, at which time the culture medium was replaced with serum-free DMEM. The cells were incubated for 24 h with either 10 μg/ml TNF-α or 10 μg/ml IL-1β. Total RNA was isolated and 1 μg of RNA was reverse transcribed into complementary (c)DNA according to the manufacturer’s instructions. The cDNA was used as the template for PCR amplification on an ABI StepOnePlus real-time PCR thermocycler (Applied Biosystems, Foster City, CA, USA) using SYBR Green PCR reagent (Applied Biosystems) and primers specific for TWEAK, TNFRSF12A, TNFA, TNF receptor (TNFR)1, TNFR2, glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
In separate experiments, PCR was performed using TaqMan universal PCR master mix to quantitatively assess the transcript levels of IL6, IL8, MCP1, ICAM1, MMP1, MMP2, MMP7, MMP9, and GAPDH in cell samples using the primers shown in S2 Table. PCR reactions were performed in triplicate; target gene expression levels were normalized to that of GAPDH, and the results are expressed as the fold change in cycle threshold (Ct) value relative to the control group as determined with the 2−ΔΔCt method [22]. Data were included in the analysis only if Ct was < 35. Results from at least three GO samples were compared with mean values of three normal control samples.
Western blotting
Western blot analysis was performed as previously described [21] using antibodies against TWEAK, Fn14, ERK, JNK, PI3K, v-Akt murine thymoma viral oncogene (Akt), and NF-κB (Cell Signaling Technology, Danvers, MA, USA). TWEAK and Fn14 expression was measured in orbital fibroblasts stimulated with IL-1β and TNF-α (10 ng/ml) for various times. To evaluate the role of TWEAK in inflammatory signaling, orbital fibroblasts were treated with TWEAK for different times and the relative amount of each protein was quantified by densitometric analysis of immunoreactive bands using ImageJ software (National Institutes of Health, Bethesda, MD, USA), with values normalized to that of the β-actin signal of the same sample.
Enzyme-linked immunosorbent assay (ELISA)
Proinflammatory cytokine, hyaluronan, and TWEAK levels in the culture supernatant of confluent orbital fibroblasts or in serum were determined using commercially available ELISA kits (IL-6, IL-8, MCP-1, and hyaluronan: R&D Systems; TWEAK: Bender Medsystems, Vienna, Austria) according to the manufacturers’ instructions. To investigate whether TWEAK-induced cytokine production is dependent on Fn14, cells were incubated with the Fn14-specific mAb ITEM4 (2.5 μg/ml) prior to stimulation. For comparison, GO cells were exposed to the signaling pathway inhibitors PD98059, SP600125, SB203580, and LY294002 (20 μM) and SC514 (10 μM) for 1 h prior to stimulation with TWEAK. The production of hyaluronan induced by TWEAK was analyzed in the same manner. Hyaluronan concentration in the sample was determined from a standard binding curve generated with known amounts of hyaluronan. Samples were diluted 1:10 before analysis, and the mean value of triplicate samples is reported.
To analyze serum level of TWEAK, blood samples were drawn into test tubes containing 10% (v/v) sodium citrate. Platelet-free plasma was obtained by centrifugation at 3000 × g for 15 min at room temperature and stored at −80°C until analysis. Serum level of TWEAK protein (pg/ml) was measured using a commercial ELISA kit. Each sample was tested three times. All serum samples were tested in the same assay.
Statistical analysis
Experiments were performed using cell samples from different donors, which were assayed in duplicate. The mean and standard deviation were calculated from normalized measurements of each mRNA or protein from multiple (at least three) samples harvested from different individuals. Data from a single cell group at different drug concentrations and incubation times were evaluated with the Student t test or by analysis of variance using SPSS v.20.0 software (SPSS Inc., Chicago, IL, USA). Pearson’s correlation coefficient was used to analyze the relationship between serum TWEAK concentration, CAS, and TBII level. A P value < 0.05 was considered statistically significant.
Results
Expression of TWEAK and its receptor in orbital tissue and fibroblasts
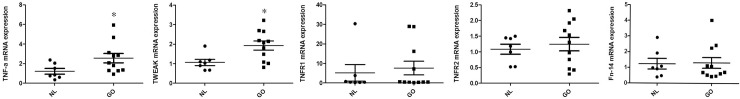
To evaluate the expression of TWEAK and its receptor in GO, orbital tissue explants were obtained from GO patients (n = 11) and non-GO healthy controls (n = 7), and relative TNFA, TWEAK, TNFR1, TNFR2, and TNFRSF12A mRNA levels were analyzed by real-time PCR. TWEAK and TNFA transcripts were upregulated in GO as compared to non-GO control tissue (P < 0.05; Fig 1). There were no differences in the levels of other receptors or of TNFRSF12A between the two groups, and TNFR1 was expressed at a similarly low level in all samples.
Fig 1. TNFA, TWEAK, TNFR1, TNFR2, and TNFRSF12A (Fn14) expression in GO and non-GO orbital tissues.
Transcript levels of TNFA as well as TWEAK and its receptors TNFR1, TNFR2, and TNFRSF12A (Fn14) were compared between GO (n = 11) and non-GO (n = 7) orbital tissues by real-time PCR. TNFA and TWEAK were upregulated in GO as compared to non-GO tissue. *P < 0.05 vs. non-GO control. NL, normal non-GO subjects.
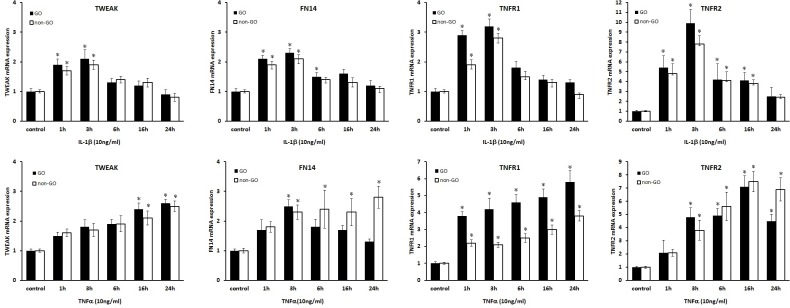
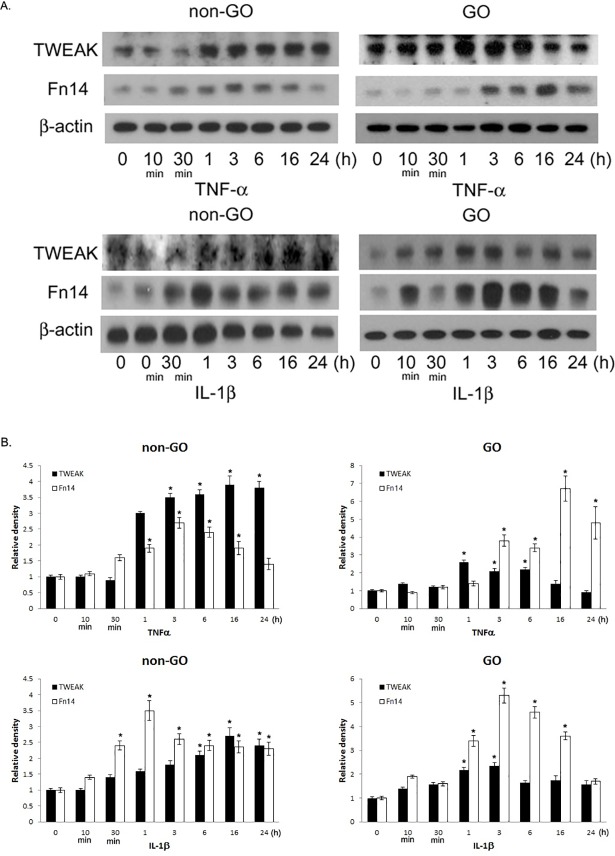
The expression of TWEAK and its receptors was assessed under inflammatory conditions in GO (n = 3) and non-GO (n = 3) orbital fibroblast cultures following stimulation with IL-1β or TNF-α by real time-PCR and western blot analyses. Treatment with IL-1β or TNF-α increased TWEAK, TNFR1, TNFR2, and Fn14 mRNA (Fig 2) levels (P < 0.05) and TWEAK and Fn14 protein levels in a time-dependent manner in both GO and non-GO cells (Fig 3).
Fig 2. Effect of IL-1β and TNF-α on TWEAK and Fn14 expression in GO and non-GO orbital fibroblasts.
Cultured GO (n = 3, black columns) and non-GO (n = 3, white columns) orbital fibroblasts were treated with IL-1β (10 ng/ml, first row) or TNF-α (10 ng/ml, second row) for 1, 3, 6, 16, or 24 h. Data in the columns represent the mean fold difference in relative mRNA level ± standard deviation of triplicate measurements. *P < 0.05 vs. untreated cells.
Fig 3. Effect of IL-1β and TNF-α on TWEAK and Fn14 protein levels in GO and non-GO orbital fibroblasts.
Cultured GO (n = 3) and non-GO (n = 3) orbital fibroblasts were treated with IL-1β (10 ng/ml) or TNF-α (10 ng/ml) from 10 min to 24 h. TWEAK and Fn14 protein levels were evaluated by western blotting. (A) Representative gel images. (B) Relative amount of each protein quantified by densitometric analysis of immunoreactive bands using ImageJ software and normalized to the level of β-actin in the same sample (B). Data in the columns represent the mean relative density ratio ± standard deviation of three experiments. *P < 0.05 vs. untreated cells.
Effect of rTWEAK on pro-inflammatory cytokine production
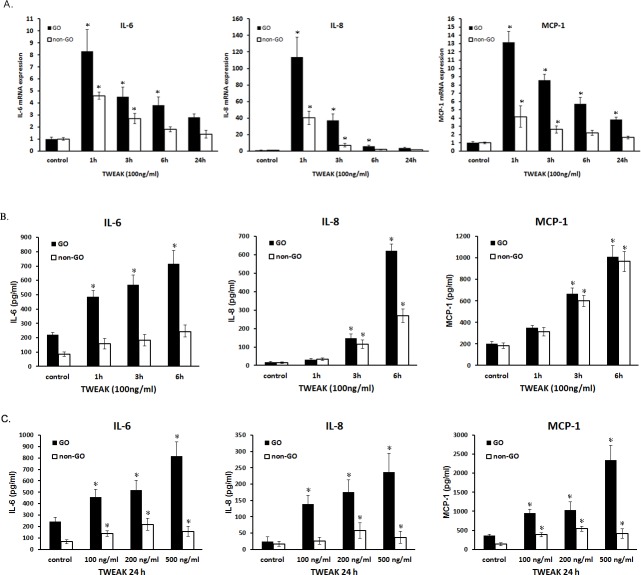
The expression of pro-inflammatory cytokines including IL-6, IL-8, and MCP-1—which are implicated in the pathogenesis of GO—was examined at the transcript and protein levels by real-time PCR and ELISA, respectively (Fig 4). Treatment with rTWEAK increased IL6, IL8, and MCP1 mRNA (Fig 4A) and protein (Fig 4B and 4C) levels in GO cells relative to non-GO cells in a time- and dose-dependent manner.
Fig 4. Effect of rTWEAK on mRNA and protein levels of IL-6, IL-8, and MCP-1 in GO and non-GO fibroblasts.
GO (n = 3, black columns) and non-GO (n = 3, white columns) orbital fibroblasts were treated with rTWEAK. (A, B) IL6, IL8, and MCP1 mRNA levels were quantified by real-time PCR (A), and protein levels were quantified by ELISA (B, C). Data in columns represent mean ± standard deviation. *P < 0.05 vs. untreated control cells.
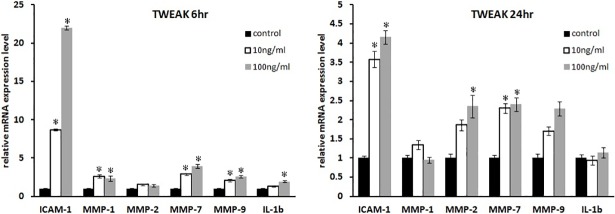
The mRNA expression of other cytokines including ICAM1, IL1B, and MMPs were analyzed in rTWEAK-treated GO cells by real-time PCR (Fig 5). ICAM1, MMP1, MMP2, MMP7, and IL1B transcripts were upregulated in a dose-dependent manner after 6 h of rTWEAK stimulation (P < 0.05; Fig 5A). In addition, ICAM1, MMP2, and MMP7 transcript levels were increased after 24 h of rTWEAK treatment (P < 0.05; Fig 5B).
Fig 5. Effect of rTWEAK on ICAM1, MMP1, MMP2, MMP7, MMP9, and IL1B mRNA levels in GO cells.
(A, B) GO orbital fibroblasts (n = 3) were cultured and treated with rTWEAK (10 and 100 ng/ml) for 6 h (A) and 24 h (B). ICAM1, MMP1, MMP2, MMP7, MMP7, MMP9, and IL1B mRNA levels were evaluated by real-time PCR. Data in columns represent the mean fold difference in relative mRNA level ± standard deviation from triplicate measurements. *P < 0.05 vs. untreated control cells.
Involvement of Fn14 and intracellular signaling pathways in TWEAK-mediated induction of pro-inflammatory cytokine production
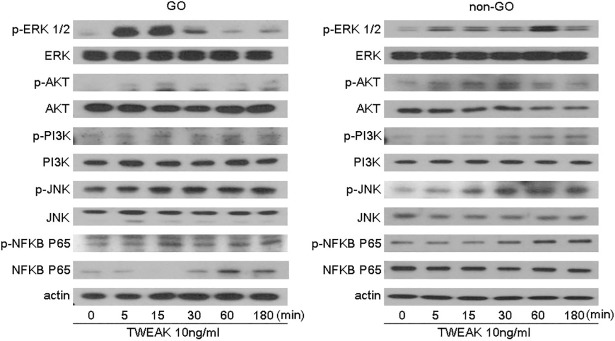
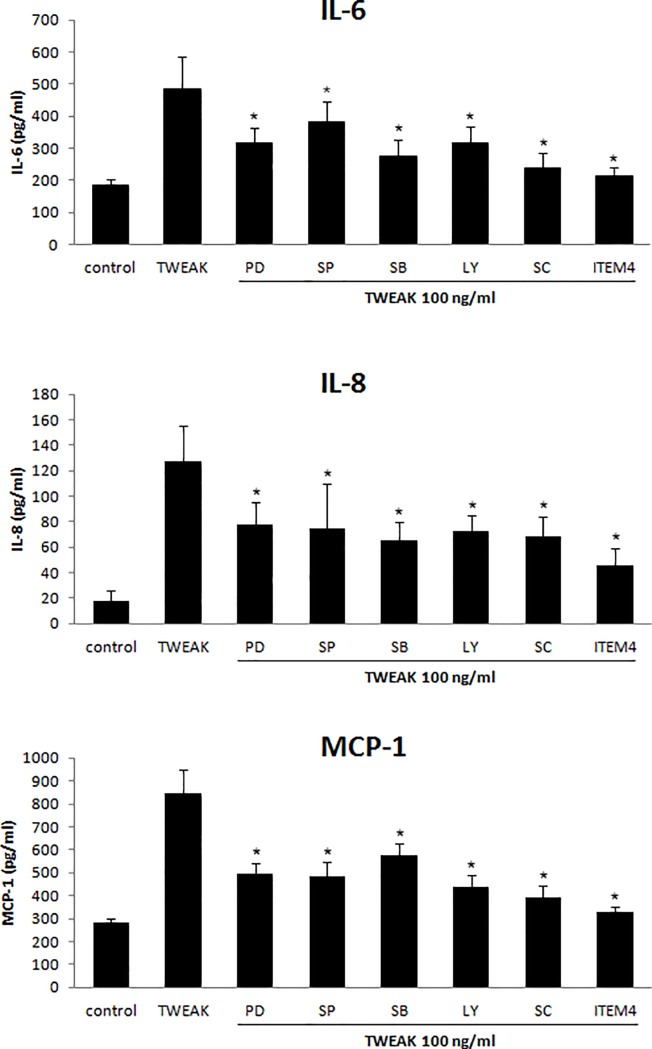
TWEAK (10 ng/ml) treatment induced the expression of phosphorylated (p-)ERK1/2, p-Akt, p-PI3K, p-JNK, and p-NF-κB p65 within 3 h in both GO and non-GO cells (Fig 6). Pretreatment with 2.5 μg/ml anti-Fn14 mAb (ITEM4) for 1 h or with inhibitors of ERK, JNK, p38 MAPK, PI3K, and NF-κB signaling reduced IL-6, IL-8, and MCP-1 protein expression induced by rTWEAK (100 ng/ml, 24 h) in GO cells (n = 3) (Fig 7). Fn14 blockade most potently suppressed rTWEAK-induced cytokine production. These results indicate that Fn14—a specific TWEAK receptor—and other signaling proteins mediate the inflammatory effect of TWEAK in GO orbital fibroblasts.
Fig 6. Effect of rTWEAK treatment on intracellular signaling in GO and non-GO cells.
Cells were treated with rTWEAK (10 ng/ml) for various times and cell lysates were analyzed by western blotting to assess p-ERK 1/2, p-Akt, p-PI3K, p-JNK, and p-NF-κB p65 levels, with β-actin serving as a loading control.
Fig 7. Role of Fn14 and intracellular signaling pathways in rTWEAK-induced pro-inflammatory cytokine expression.
IL-6, IL-8, and MCP-1 protein levels in GO orbital fibroblasts (n = 3) pretreated with ERK kinase (PD98059, 20 μM), JNK (SP600125, 20 μM), p38 MAPK (SB203580, 20 μM), PI3K (LY294002, 20 μM), NF-κB p65 (SC514, 10 μM), and Fn14 (ITEM4, 2.5 μg/ml) inhibitor for 1 h followed by rTWEAK treatment (100 ng/ml, 24 h) were measured by ELISA. Data in columns represent mean ± standard deviation of three experiments. *P < 0.05 vs. rTWEAK-treated cells without inhibitor pretreatment.
Effect of TWEAK on hyaluronan production
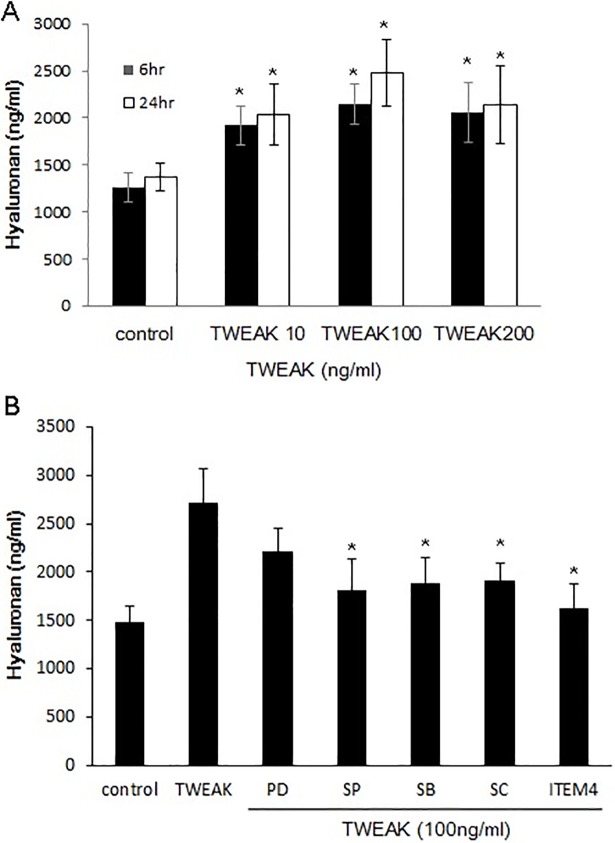
We investigated the effect of TWEAK on the production of hyaluronan, which is an important step in the pathogenesis of GO. The release of hyaluronan into the culture medium following treatment with 10–200 ng/ml TWEAK for 6 and 24 h was measured by ELISA. Hyaluronan level was increased in GO cells upon stimulation with rTWEAK (Fig 8A). Pretreatment with a mAb against Fn14 (ITEM4, 2.5 μg/ml) suppressed rTWEAK (100 ng/ml, 24 h)-induced hyaluronan release (Fig 8B). Inhibition of JNK, p38 MAPK, PI3K, and NF-κB p65 (20 μM, 1 h) pathways similarly reduced hyaluronan production.
Fig 8. Effect of Fn14 inhibitor on rTWEAK-induced hyaluronan production in GO cells.
Hyaluronan (ng/ml) level in GO orbital fibroblasts (n = 3) was measured by ELISA after treatment with rTWEAK (10, 100 and 200 ng/ml) for 6 and 24 h. Cells (n = 3) were pretreated with ERK kinase (PD98059, 20 μM), JNK (SP600125, 20 μM), p38 MAPK (SB203580, 20 μM), NF-κB p65 (SC514, 10 μM), and Fn14 (ITEM4, 2.5 μg/ml) inhibitor for 1 h followed by rTWEAK treatment (100 ng/ml, 24 h). Data in columns represent mean ± standard deviation of three experiments. *P < 0.05 vs. rTWEAK-treated cells without inhibitor pretreatment.
TWEAK protein level is elevated in the serum of GO patients
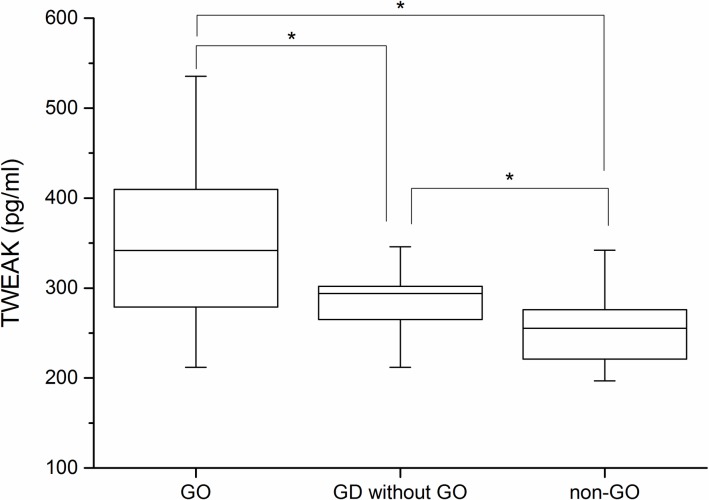
We measured serum TWEAK levels in GO patients (n = 56), GD patients without GO (n = 35), and age- and sex-matched healthy control subjects (n = 39). Mean age was similar among groups (GO patients, 36.17 years; GD patients without GO, 34.34 years; and healthy subjects, 32.41 years). Mean serum levels of TWEAK were higher in GO patients (341.86 ± 86.3 pg/ml) than in GD patients without GO (294.09 ± 41.44 pg/ml) (P < 0.05), who had higher levels than healthy subjects (255.33 ± 39.38 pg/ml) (P < 0.05; Fig 9).
Fig 9. Serum levels of TWEAK in GO patients, GD patients without GO, and normal subjects.
Serum levels of TWEAK (pg/ml) were higher in GO patients (n = 56) than in GD patients without GO (n = 35) and healthy subjects (n = 39). Data represent median and interquartile range of values determined by ELISA. *P < 0.05 vs. each group.
We examined the correlation between TWEAK level, CAS, and TSHR Ab level by Pearson’s correlation analysis and found that serum TWEAK level was correlated with CAS (r = 0.629, P < 0.001) and TBII level (r = 0.659 P < 0.001).
Discussion
The results presented here demonstrate for the first time that TWEAK activates inflammation and hyaluronan production in GO fibroblasts, and that blocking the TWEAK receptor Fn14 abrogates these effects. Cytokine release and hyaluronan production in orbital fibroblasts are important steps in the initiation of exophthalmos and myopathy in the pathogenesis of GO. We showed here that TNF-α and TWEAK levels were higher in GO than in non-GO orbital tissues. Pro-inflammatory cytokines such as IL-1β and TNF-α induced the expression of TWEAK and its receptors TNFR-1, TNFR-2, and Fn14 in GO orbital fibroblasts. Conversely, rTWEAK treatment increased the levels of the pro-inflammatory cytokines IL-6, IL-8, and MCP-1, MMPs, and adhesion molecules such as ICAM-1 in orbital fibroblasts. In addition, hyaluronan production by GO fibroblasts was enhanced by treatment with TWEAK, an effect that was abolished by pretreatment with anti-Fn14 mAb. Fn14—which is induced in multiple cell types during tissue injury—along with some intracellular signaling pathways were involved in TWEAK-induced pro-inflammatory cytokine production. These findings provide evidence for the role of TWEAK/Fn14 signaling in GO pathogenesis.
Serum level of TWEAK was elevated in GO patients as compared to GD patients without GO and non-GO subjects; it was also positively correlated with CAS—a well-known indicator of inflammation—and TBII, which predicts GO activity or severity.[23] Similar TWEAK levels were observed between GO patients with and without radioiodine treatment, (357.33 vs. 337.4 pg/ml; P = 0.484). We presume that the radioiodine did not influence serum TWEAK level since it was administered at least 6 months prior to study enrollment. It was reported that serum TWEAK level was positively correlated with disease activity score-28 in RA patients [19] and was associated with a lower incidence of pulmonary fibrosis and better pulmonary function in systemic sclerosis patients.[24] Although the biological activity of TWEAK is complex, it is known to be activated in inflammatory autoimmune disease and may serve as a reliable diagnostic and prognostic biomarker. Identifying new biomarkers and therapeutic targets other than TSHR for GD and GO by combining microRNA and protein sequencing [25] can improve our understanding of the pathogenesis of these disorders and lead to the development of effective treatments.
Fn14 is a member of the TNFR family [26] that was identified as a functional TWEAK receptor in a cDNA expression library screen [26]. Fn14 is expressed at a low level in various cell types, and is induced under conditions of stress including inflammation. Several growth factors, cytokines, and interleukins are known to induce Fn14 expression in injured endothelial cells, vascular smooth muscle cells, monocytes, and macrophages but not in T and B lymphocytes [26,27]. TWEAK and Fn14 have been implicated in a variety of pathologies and their extracellular localization makes them accessible pharmacological targets, with recombinant soluble variants of or antibodies against these molecules showing high selectivity [24]. It was recently demonstrated using an anti-human Fn14 mAb that TWEAK-induced cell proliferation, migration, and death was mediated by Fn14 [28–30]. TWEAK-specific antibodies that block binding to Fn14 were generated by fusing the ectodomain of Fn14 and the Fc domain of human IgG1. We found that blocking Fn14 using anti-Fn14 mAb suppressed rTWEAK-induced cytokine production and hyaluronan release in cultured GO orbital fibroblasts. Thus, TWEAK/Fn14 signaling plays a protective role in the acute stage of GO-associated inflammation via suppression of cytokine and chemokine release in orbital fibroblasts.
TWEAK activates several signaling pathways that participate in the inflammatory response in injured tissues. NF-κB plays a key role in TWEAK-induced inflammation [31–33]. TWEAK also activates MAPK, ERK, JNK, and p38 pathways [17,34],[35] as well as PI3K/AKT signaling in different cell types. TWEAK induction of JNK is related to activator protein-1 activation [36]. Consistent with these reports, we found that TWEAK induced the phosphorylation of ERK, Akt/PI3K, JNK, and NF-κB p65 in orbital fibroblasts. Furthermore, inhibiting these signaling proteins blocked TWEAK-induced IL-6, IL-8, and MCP-1 expression and hyaluronan production, which was similar to the suppressive effect of Fn14-blocking Ab, indicating that TWEAK/FN14 signaling in the context of GO involves the ERK, Akt/PI3K, JNK, and NF-κB p65 pathways.
Conclusions
The present study showed that TWEAK is expressed in GO orbital fibroblasts and may contribute to orbital inflammation by inducing pro-inflammatory cytokine secretion. TWEAK level was elevated in the serum of GO patients as compared to GD patients without GO and non-GO subjects, and was positively correlated with clinical inflammation and TSHR Ab titer, indicating that TWEAK is a potential biomarker for diagnosing inflammatory GO. In addition, we showed that inhibiting the TWEAK receptor Fn14 can alleviate TWEAK-mediated GO pathology. Thus, our findings provide evidence that targeting TWEAK/Fn14 signaling may be an effective therapeutic strategy for GO treatment.
Supporting information
GD, Graves' disease; GO, Graves' orbitopathy; SD, standard deviation; TSH, thyroid-stimulating hormone; TBII, thyrotropin binding inhibitory immunoglobulin.
(DOCX)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (NRF-2017R1A2B4009565). JSY received the grant. (www.nrf.re.kr). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Wiersinga WM, Smit T, van der Gaag R, Koornneef L. Temporal relationship between onset of Graves' ophthalmopathy and onset of thyroidal Graves' disease. J Endocrinol Invest. 1988;11: 615–619. 10.1007/BF03350193 [DOI] [PubMed] [Google Scholar]
- 2.Lazarus JH. Epidemiology of Graves' orbitopathy (GO) and relationship with thyroid disease. Best Pract Res Clin Endocrinol Metab. 2012;26: 273–279. 10.1016/j.beem.2011.10.005 [DOI] [PubMed] [Google Scholar]
- 3.Feldon SE, Park DJ, O'Loughlin CW, Nguyen VT, Landskroner-Eiger S, Chang D, et al. Autologous T-lymphocytes stimulate proliferation of orbital fibroblasts derived from patients with Graves' ophthalmopathy. Investigative ophthalmology & visual science. 2005;46: 3913–3921. [DOI] [PubMed] [Google Scholar]
- 4.Grubeck-Loebenstein B, Trieb K, Sztankay A, Holter W, Anderl H, Wick G. Retrobulbar T cells from patients with Graves' ophthalmopathy are CD8+ and specifically recognize autologous fibroblasts. J Clin Invest. 1994;93: 2738–2743. 10.1172/JCI117289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Otto EA, Ochs K, Hansen C, Wall JR, Kahaly GJ. Orbital tissue-derived T lymphocytes from patients with Graves' ophthalmopathy recognize autologous orbital antigens. J Clin Endocrinol Metab. 1996;81: 3045–3050. 10.1210/jcem.81.8.8768872 [DOI] [PubMed] [Google Scholar]
- 6.Pappa A, Lawson JM, Calder V, Fells P, Lightman S. T cells and fibroblasts in affected extraocular muscles in early and late thyroid associated ophthalmopathy. Br J Ophthalmol. 2000;84: 517–522. 10.1136/bjo.84.5.517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuriyan AE, Phipps RP, Feldon SE. The eye and thyroid disease. Curr Opin Ophthalmol. 2008;19: 499–506. 10.1097/ICU.0b013e3283131557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bahn RS. Clinical review 157: Pathophysiology of Graves' ophthalmopathy: the cycle of disease. J Clin Endocrinol Metab. 2003;88: 1939–1946. 10.1210/jc.2002-030010 [DOI] [PubMed] [Google Scholar]
- 9.Iyer S, Bahn R. Immunopathogenesis of Graves' ophthalmopathy: the role of the TSH receptor. Best Pract Res Clin Endocrinol Metab. 2012;26: 281–289. 10.1016/j.beem.2011.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saas P, Boucraut J, Walker PR, Quiquerez AL, Billot M, Desplat-Jego S, et al. TWEAK stimulation of astrocytes and the proinflammatory consequences. Glia. 2000;32: 102–107. [PubMed] [Google Scholar]
- 11.Wong YK, Tang KT, Wu JC, Hwang JJ, Wang HS. Stimulation of hyaluronan synthesis by interleukin-1beta involves activation of protein kinase C betaII in fibroblasts from patients with Graves' ophthalmopathy. J Cell Biochem. 2001;82: 58–67. [DOI] [PubMed] [Google Scholar]
- 12.Burkly LC, Michaelson JS, Hahm K, Jakubowski A, Zheng TS. TWEAKing tissue remodeling by a multifunctional cytokine: role of TWEAK/Fn14 pathway in health and disease. Cytokine. 2007;40: 1–16. 10.1016/j.cyto.2007.09.007 [DOI] [PubMed] [Google Scholar]
- 13.Winkles JA. The TWEAK-Fn14 cytokine-receptor axis: discovery, biology and therapeutic targeting. Nat Rev Drug Discov. 2008;7: 411–425. 10.1038/nrd2488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campbell S, Michaelson J, Burkly L, Putterman C. The role of TWEAK/Fn14 in the pathogenesis of inflammation and systemic autoimmunity. Front Biosci. 2004;9: 2273–2284. [DOI] [PubMed] [Google Scholar]
- 15.Chicheportiche Y, Chicheportiche R, Sizing I, Thompson J, Benjamin CB, Ambrose C, et al. Proinflammatory activity of TWEAK on human dermal fibroblasts and synoviocytes: blocking and enhancing effects of anti-TWEAK monoclonal antibodies. Arthritis Res. 2002;4: 126–133. 10.1186/ar388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harada N, Nakayama M, Nakano H, Fukuchi Y, Yagita H, Okumura K. Pro-inflammatory effect of TWEAK/Fn14 interaction on human umbilical vein endothelial cells. Biochem Biophys Res Commun. 2002;299: 488–493. [DOI] [PubMed] [Google Scholar]
- 17.Hosokawa Y, Hosokawa I, Ozaki K, Nakae H, Matsuo T. Proinflammatory effects of tumour necrosis factor-like weak inducer of apoptosis (TWEAK) on human gingival fibroblasts. Clin Exp Immunol. 2006;146: 540–549. 10.1111/j.1365-2249.2006.03233.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown SA, Cheng E, Williams MS, Winkles JA. TWEAK-independent Fn14 self-association and NF-kappaB activation is mediated by the C-terminal region of the Fn14 cytoplasmic domain. PLoS One. 2013;8: e65248 10.1371/journal.pone.0065248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park MC, Chung SJ, Park YB, Lee SK. Relationship of serum TWEAK level to cytokine level, disease activity, and response to anti-TNF treatment in patients with rheumatoid arthritis. Scand J Rheumatol. 2008;37: 173–178. 10.1080/03009740801898608 [DOI] [PubMed] [Google Scholar]
- 20.Kamata K, Kamijo S, Nakajima A, Koyanagi A, Kurosawa H, Yagita H, et al. Involvement of TNF-like weak inducer of apoptosis in the pathogenesis of collagen-induced arthritis. J Immunol. 2006;177: 6433–6439. [DOI] [PubMed] [Google Scholar]
- 21.Yoon JS, Lee HJ, Choi SH, Chang EJ, Lee SY, Lee EJ. Quercetin inhibits IL-1beta-induced inflammation, hyaluronan production and adipogenesis in orbital fibroblasts from Graves' orbitopathy. PLoS One.6: e26261 10.1371/journal.pone.0026261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25: 402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 23.Jang SY, Shin DY, Lee EJ, Lee SY, Yoon JS. Relevance of TSH-receptor antibody levels in predicting disease course in Graves' orbitopathy: comparison of the third-generation TBII assay and Mc4-TSI bioassay. Eye (Lond). 2013;27: 964–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wajant H. The TWEAK-Fn14 system as a potential drug target. Br J Pharmacol. 2013;170: 748–764. 10.1111/bph.12337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang L, Masetti G, Colucci G, Salvi M, Covelli D, Eckstein A, et al. Combining micro-RNA and protein sequencing to detect robust biomarkers for Graves' disease and orbitopathy. Sci Rep. 2018;8: 8386 10.1038/s41598-018-26700-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiley SR, Cassiano L, Lofton T, Davis-Smith T, Winkles JA, Lindner V, et al. A novel TNF receptor family member binds TWEAK and is implicated in angiogenesis. Immunity. 2001;15: 837–846. [DOI] [PubMed] [Google Scholar]
- 27.Maecker H, Varfolomeev E, Kischkel F, Lawrence D, LeBlanc H, Lee W, et al. TWEAK attenuates the transition from innate to adaptive immunity. Cell. 2005;123: 931–944. 10.1016/j.cell.2005.09.022 [DOI] [PubMed] [Google Scholar]
- 28.Burkly LC, Dohi T. The TWEAK/Fn14 pathway in tissue remodeling: for better or for worse. Adv Exp Med Biol. 2011;691: 305–322. 10.1007/978-1-4419-6612-4_32 [DOI] [PubMed] [Google Scholar]
- 29.Kawakami Y, Yumoto K, Kawakami T. An improved mouse model of atopic dermatitis and suppression of skin lesions by an inhibitor of Tec family kinases. Allergol Int. 2007;56: 403–409. 10.2332/allergolint.O-07-486 [DOI] [PubMed] [Google Scholar]
- 30.Wilhelm A, Shepherd EL, Amatucci A, Munir M, Reynolds G, Humphreys E, et al. Interaction of TWEAK with Fn14 leads to the progression of fibrotic liver disease by directly modulating hepatic stellate cell proliferation. J Pathol. 2016;239: 109–121. 10.1002/path.4707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poveda J, Tabara LC, Fernandez-Fernandez B, Martin-Cleary C, Sanz AB, Selgas R, et al. TWEAK/Fn14 and Non-Canonical NF-kappaB Signaling in Kidney Disease. Front Immunol. 2013;4: 447 10.3389/fimmu.2013.00447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Enwere EK, Lacasse EC, Adam NJ, Korneluk RG. Role of the TWEAK-Fn14-cIAP1-NF-kappaB Signaling Axis in the Regulation of Myogenesis and Muscle Homeostasis. Front Immunol. 2014;5: 34 10.3389/fimmu.2014.00034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roos C, Wicovsky A, Muller N, Salzmann S, Rosenthal T, Kalthoff H, et al. Soluble and transmembrane TNF-like weak inducer of apoptosis differentially activate the classical and noncanonical NF-kappa B pathway. J Immunol. 2010;185: 1593–1605. 10.4049/jimmunol.0903555 [DOI] [PubMed] [Google Scholar]
- 34.Burkly LC, Michaelson JS, Zheng TS. TWEAK/Fn14 pathway: an immunological switch for shaping tissue responses. Immunol Rev. 2011;244: 99–114. 10.1111/j.1600-065X.2011.01054.x [DOI] [PubMed] [Google Scholar]
- 35.Li H, Mittal A, Paul PK, Kumar M, Srivastava DS, Tyagi SC, et al. Tumor necrosis factor-related weak inducer of apoptosis augments matrix metalloproteinase 9 (MMP-9) production in skeletal muscle through the activation of nuclear factor-kappaB-inducing kinase and p38 mitogen-activated protein kinase: a potential role of MMP-9 in myopathy. J Biol Chem. 2009;284: 4439–4450. 10.1074/jbc.M805546200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dogra C, Hall SL, Wedhas N, Linkhart TA, Kumar A. Fibroblast growth factor inducible 14 (Fn14) is required for the expression of myogenic regulatory factors and differentiation of myoblasts into myotubes. Evidence for TWEAK-independent functions of Fn14 during myogenesis. J Biol Chem. 2007;282: 15000–15010. 10.1074/jbc.M608668200 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
GD, Graves' disease; GO, Graves' orbitopathy; SD, standard deviation; TSH, thyroid-stimulating hormone; TBII, thyrotropin binding inhibitory immunoglobulin.
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.