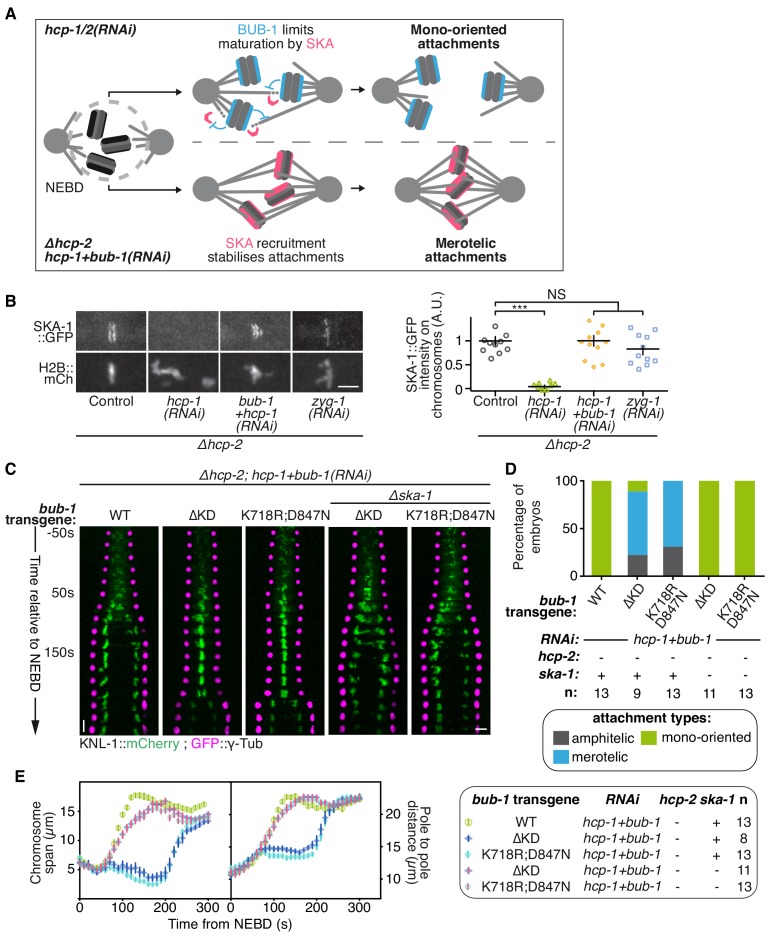
Figure 5. BUB-1 inhibits biorientation in absence of HCP-1/2CENP-F by preventing SKA complex recruitment.
(A) Schematics of the potential mechanism for chromosome biorientation inhibition by BUB-1 in absence of HCP-1/2CENP-F. BUB-1 limits attachment maturation by the SKA complex, leading to the incapacity for chromosomes to birorient when microtubules are short and non-dynamic. Co-depleting BUB-1 restores SKA complex recruitment, allowing the strengthening of attachments and therefore the establishment of biorientation even when microtubules are short and non-dynamic. (B) Left: Representative images from time-lapse movies showing the localization of SKA-1::GFP on chromosomes (H2B::mCherry) in the indicated conditions at metaphase. Right: Quantification of the GFP signal on chromosomes at metaphase. Kruskall Wallis tests with Dunn’s correction for multiplicity were used to assess significance (hcp-1(RNAi) p = 0,0006, hcp-1 +bub-1(RNAi) p > 0.9999, zyg-1(RNAi) p > 0,9999). (C) Kymographs generated from embryos expressing GFP::γ-Tub and KNL-1::mCherry for the indicated conditions. (D) Quantification of the percentage of embryos with chromosomes engaged in amphitelic, merotelic and mono-oriented attachments in the indicated conditions. (E) Chromosome span and pole to pole distance as functions of time after NEBD for the indicated conditions. Error bars represent the SEM. Horizontal scale bars, 5 μm; Vertical scale bar, 20 s.