Abstract
The vitamin D receptor is expressed in most tissues of the body – and the cancers that arise from those tissues. The vitamin D signaling pathway is active in those tissues and cancers. This is at least consistent with the hypothesis that perturbing this signaling may have a favorable effect on the genesis and growth of cancers. Epidemiologic data indicate that vitamin D signaling may be important in the initiation and outcome of a number of types of cancer. Many studies have shown that calcitriol (1,25 dihydroxycholecalciferol) and other vitamin D compounds have antiproliferative, pro-apoptotic, anti-cell migration and antiangiogenic activity in a number of preclinical studies in many different cancer types. Unfortunately, the assessment of the activity of calcitriol or other vitamin D analogues in the treatment of cancer, as single agents or in combination with other anticancer agents has been stymied by the failure to adhere to commonly accepted principles of drug development and clinical trials conduct.
Keywords: Vitamin D, Cancer, Calcitriol
Highlights
-
•
Many studies indicate that vitamin D compounds have cancer prevention and treatment properties in vitro and in vivo cancer models and epidemiologic studies note the association between low vitamin D levels and cancer occurrence and unfavorable outcome.
-
•
Many clinical trials in cancer patients have been done to define safety and efficacy of calcitriol and analogues and several trials suggest clinical benefit.
-
•
Two randomized trials of calcitriol + chemotherapy (docetaxel) in men with prostate cancer entered >1150 patients.
-
•
These trials did not adhere to principles of optimal trial design (choice of optimal dose of drug and balanced randomization) and the failure to execute on these findings is a missed opportunity.
1. Case report
The patient is a 67 yo white man with prostate cancer, diagnosed 7 years previously (T2bN0M0, Gleason 4 + 3 = 7 cancer involving ~30% of the prostate which was removed at robotic prostatectomy. Despite prostatectomy the patient's prostate specific antigen blood level became detectable 24 months following surgery. Despite irradiation to the prostatic fossa and then androgen deprivation therapy (ADT) with leuprolide + bicalutamide 5 years following prostatectomy PSA was 110 ng/mL and radiographs demonstrated metastases to multiple sites in the boney skeleton (T6 & L1,2 vertebral bodies, both iliac wings, the pubic ramus and the 10th right rib). No visceral or soft tissue metastases were present. Antiandrogen withdrawal resulted in a drop in PSA to 45 ng/mL, but 1 year later the PSA was rising again (78 ng/mL), the patient was asymptomatic and the radiographs demonstrated bone disease, but no visceral or soft tissue metastases. After obtaining informed consent the patient entered an ongoing phase II trial of high dose oral calcitriol + dexamethasone. Oral calcitriol was administered weekly, Monday, Tuesday, and Wednesday (MTW), at a dose of 8 μg, for 1 month, 10 μg every MTW for 1 month, and at 12 μg every MTW thereafter. Dexamethasone at a dose of 4 mg was administered each Sunday, and MTW weekly. Calcium and creatinine were determined weekly and radiographs of the urinary tract were performed every 3 months to monitor for urinary tract stone formation. On day 56 of therapy the PSA had fallen to 30 ng/mL, the radiographs showed no new metastases, stable bone disease and no urinary tract stones. Weekly blood calcium and creatinine concentrations were always within the normal range and no biochemical or subjective toxicity occurred. The patient continued on therapy for a total of 14 months and then with continuingly stable disease, (PSA 42 ng/mL) the patient elected to cease therapy and was followed receiving only ADT. Is this indication of the therapeutic effect of high dose calcitriol in prostate cancer? (Trump et al., 2006)
2. Introduction
Critical experiments, reported in the 1970s and early 1980s, form the basis for the hypothesis that vitamin D compounds can be used to inhibit the development and progression of several types of cancer:
-
•
treatment with vitamin D compounds was shown to inhibit the development of cancer in a carcinogen-induced model in the hamster
-
•
the vitamin D receptor (VDR) was detected in numerous histotypes of human cancer cells
-
•
treatment of cancer cells with calcitriol (1,25dihydroxycholecalciferol) in vitro was shown to cause growth arrest (Potter et al., 1985; Moqattash et al., 1985; Freake et al., 1984; Sato et al., 1984; Kuroki et al., 1983; Frampton et al., 1983; Frankel et al., 1983; Mccarthy et al., 1983; Honma et al., 1983; Sato et al., 1982; Freake & Macintyre, 1982; Tanaka et al., 1982; Colston et al., 1982; Frampton et al., 1982; Eisman et al., 1981; Sher et al., 1981; Miyaura et al., 1981; Abe et al., 1981; Colston et al., 1981; Eisman et al., 1980a; Fry et al., 1980; Eisman et al., 1980b; Manolagas et al., 1980; Eisman et al., 1980c; Eisman et al., 1979; Murphy et al., 1979; Rubin & Levij, 1973).
Subsequently, hundreds of cholecalciferol analogues have been developed seeking to define more potent compounds and hoping to reduce the propensity to cause hypercalcemia, the only known toxic effect of vitamin D compounds (Van Belle et al., 2014a; González-Pardo et al., 2014; González-Pardo et al., 2013; Duffy et al., 2017; Koeffler et al., 1984; Eisman et al., 1986; Murayama et al., 1986; Ostrem et al., 1987; Haq et al., 1993; Binderup et al., 1991). This paper provides an overview of studies of calcitriol and other vitamin D compounds in the treatment of cancer, emphasizing that, while the preclinical data are convincing, clinical development has been slow and disappointing because of errors made in the basic concepts of drug development. While much of the clinical work done in developing calcitriol in cancer has been done in men with prostate cancer, this is likely due to fact that the research teams engaged in the preclinical and clinical work on calcitriol were also engaged in studies in prostate cancer. All existing data indicate that perturbation of the vitamin D signaling axis would be effective in inhibiting a wide variety of cancer types.
3. Biochemistry and molecular biology of vitamin D
Vitamin D is synthesized in the body through a complex series of steps beginning in the skin where ultraviolet light transforms 7-dehydrocholesterol into the vitamin D hormone precursor, cholecalciferol (vitamin D3). Cholecalciferol is subsequently hydroxylated in the liver (yielding 25(OH) D3) and then in the kidney to yield the most active hormone form of these compounds, 1,25 dihydroxycholecalciferol or calcitriol. “Inactivation” of calcitriol occurs primarily by 24-hydroxylation in the kidney yielding 1,24,25(OH)3 cholecalciferol. These hydroxylations are mediated primarily by cytochrome P450 (CYP) enzymes CYP2R1, CYP27B1 and CYP24A1, respectively. While the major sites of metabolism and inactivation are liver and kidneys, tumor cells as well as many other tissues throughout the body and in the microenvironment of tumor cells also metabolize these hormones (Sharifi, 2013; Ishizaki et al., 2013; JH1 et al., 2012).
1,25D3 enters cells by passive diffusion, binds to the vitamin D receptor (VDR) which heterodimerizes with the retinoid X receptor (RXR). This complex binds to promoter regions of vitamin D-responsive genes to modulate the expression of >2000 genes. The complexities of genetic variants in enzymes responsible for D3 metabolism, protein binding, partner heterodimerization and the multitude of genes modulated by the vitamin D hormone systems suggest that dissection of the role of vitamin D in cancer, as well as other diseases, will require very careful and detailed study.
4. Epidemiologic studies of vitamin D in cancer
An additional reason to examine the role of vitamin D in cancer therapy is the large number of epidemiologic studies linking vitamin D and cancer risk and outcome. Studies in colorectal, breast, lung, non-Hodgkin lymphoma, prostate and bladder cancer – to name only a few cancer types – indicate a higher risk of cancer and poor prognosis of that cancer among individuals with measured or estimated low levels of 25(OH)D3. While there is not uniform concordance among studies seeking to define an association between vitamin D and cancer causation and outcome, this work is ultimately hampered that such studies can only demonstrate “associations” between estimated or measured vitamin D levels, or genotypic variants of proteins important in vitamin D signaling and cancer risk or outcome (Feldman et al., 2014; Grant, 2012; Afzal et al., 2013; Skaaby et al., 2014; Ordóñez-Mena et al., 2013; Ordóñez-Mena et al., 2016; Yin et al., 2013; Ma et al., 2011; Garland & Gorham, 2017; Feng et al., 2017; Liu et al., 2017; Bauer et al., 2013; Gandini et al., 2011). Much greater clarity on the question of the role of vitamin D and cancer risk will be provided by the results of 2 large randomized trials of vitamin D supplementation in large populations of otherwise healthy individuals (Okereke et al., 2018; Neale et al., 2016).
5. Anticancer effects of vitamin D compounds
The biochemical changes associated with anticancer effects of calcitriol treatment of cancer cells have been extensively studied. The exact mechanisms of the antitumor effects of vitamin D compounds are incompletely understood. Calcitriol (1,25 dihydroxycholecalciferol) is the compound most carefully studied, in vitro and in vivo. Calcitriol inhibits tumor growth in association with the following biochemical effects:
-
•
Cell cycle arrest and modulation of CDK inhibitors, such as p21 and p27 (Wang et al., 1996; Simboli-Campbell et al., 1997; Verlinden et al., 1998).
-
•
Induction of apoptosis with PARP cleavage, annexin binding, increased bax/bcl-2 ratio (Bernardi et al., 2001; Welsh, 1994; James et al., 1996; Pintado et al., 1996; Narvaez & Welsh, 1997; Colston & Hansen, 2002).
-
•
Suppression of the “pro-proliferative” signaling molecules such as P-MAPK (ERK1/2), P-AKT, reduction of AKT and MEKK-1 (Johnson et al., 2006).
-
•
Induction of caspase- dependent MEK-cleavage (Alagbala et al., 2006; McGuire et al., 2001).
Each of these effects has been described to occur in multiple cancer model systems in vitro and in vivo. In addition to the aforementioned biochemical effects, the following cellular effects which may be accompanied by tumor growth suppression are well described:
-
•
Inhibition of angiogenesis (Miyata et al., 2013; Chung et al., 2009).
-
•
Inhibition of motility and invasion (Hou et al., 2016; Chen et al., 2015a).
-
•
Induction of differentiation (Olsson et al., 1983; Koeffler et al., 1985; Abe et al., 1986).
-
•
Modulation of cytokine/growth factor production by stromal and/or tumor cells (Irani et al., 2015; Chen et al., 2015b; Mohapatra et al., 2015)
In addition, considerable evidence supports the role of vitamin D signaling in immune function and inflammation. Immune dysregulation and inflammation are increasingly recognized as viable targets in cancer therapy and prevention. While the precise role of vitamin D in regulating immune function is still being defined, there are many studies demonstrating the impact of vitamin D signaling on monocyte/macrophage differentiation, T cell function and cytokine production (Gonzalez-Cao et al., 2015; Tran et al., 2017; Byun et al., 2017). Two separate studies in patients with non-Hodgkin lymphoma indicate that low levels of 25(OH)D3 are associated with poor outcome following therapy with cytotoxic chemotherapy + rituximab, an antibody which binds to CD-20 in lymphoma cells (Kelly et al., 2015; Bittenbring et al., 2014). Bittinbring et al. demonstrated that rituximab-mediated killing of lymphoma cells in vitro by an individual's monocytes was substantially enhanced by restoration of normal vitamin D levels in such individuals (Bittenbring et al., 2014).
Cyclooxygenase-2 (COX-2), the enzyme that catalyzes prostaglandin synthesis, has been extensively investigated as a target in cancer therapy and prevention (Wang et al., 2005; Zha et al., 2001; Gupta et al., 2000; Yoshimura et al., 2000; Krishnan & Feldman, 2010; Sun et al., 2018). COX-2 is overexpressed in putative cancer precursor inflammatory lesions in tissues such as breast and prostate, in a variety of cancer cell lines, as well as in macrophages and other cells in the tumor microenvironment (de Cremoux et al., 2018; Sano et al., 2018). Calcitriol regulates the expression of several genes in the prostaglandin pathway; in vitro and in vivo studies demonstrate that calcitriol + nonsteroidal anti-inflammatory agents which inhibit COX-2 potentiate the growth inhibitory effects of calcitriol (Davila-Gonzalez et al., 2017; Basudhar et al., 2017). 1,25(OH)2D analogues have also been described to suppress inflammation as well as COX-2 expression and activity either directly or indirectly (Moreno et al., 2005; Aparna et al., 2008).
Calcitriol may alter androgen metabolism in prostate cancer cells or estrogen metabolism in breast and endometrial cells, providing yet another pathway whereby tumor growth may be disrupted. CYP3A4, CYP3A5, CYP3A43, AKR1C1–3, UGT2B15/17, HSD17B2, CYP27A1 and SULT2B1b are enzymes important in cholesterol and steroid hormone metabolism; activity of these enzymes may influence estrogen receptor modulating compounds (e.g. 27-deoxycholesterol), testosterone, dehydroepiandrosterone (DHEA) and androstanediol concentrations intracellularly. Vitamin D compounds may influence the activity of these enzymes in tissues and ultimately modulate the availability of these pro-survival androgenic and estrogenic steroids. There is no conclusive evidence that vitamin D compounds modulate “intracrine” androgen or estrogen metabolism in patients, but preclinical studies are consistent with the hypothesis that this is an additional mechanism whereby 1,25(OH)2D compounds may suppress prostate tumor growth (Doherty et al., 2014; Seo et al., 2013; Maguire et al., 2012; Going et al., 2018).
6. Analogues of 1,25(OH)2D
Considerable work has been done seeking to delineate analogues of 1,25(OH)2D that may have greater antitumor activity and/or less potential to induce hypercalcemia, the only known toxic effect of vitamin D compounds. The analogues EB1089, MC903, 22-oxacalcitriol, BGP-13(a 24-chloro calcipotriene-based D3 analogue), R024–2637, 19-nor-14-epi-23-yne-1,25(OH)2D3 (TX 522, inecalcitol) and 19-nor-14,20-bisepi-23-yne-1,25(OH)2D3 (TX 527) are reported to be less likely to cause hypercalcemia than the parent compound calcitriol. Each of these analogues appears to have activity in preclinical cancer models (Skowronski et al., 1995; Campbell et al., 1997; Berkovich et al., 2013; Okamoto et al., 2012; Verlinden et al., 2000; Van Belle et al., 2014b; Ferreira et al., 2013; Verlinden et al., 2013).
For example, phase I and II trials of inecalcitol (TX522) have been completed and demonstrated that (1) 4000 μg QOD is a safe dose and (2) inecalcitol + docetaxel appears perhaps to be superior to docetaxel alone in men with castration resistant prostate cancer (Medioni et al., 2014; Medioni et al., 2011). While appealing conceptually, neither of these studies is based on any study of the biologically “optimal” dose of inecalcitol; nor was the phase II trial suggesting superior outcome sufficiently powered to be conclusive. In addition, no trials have shown clearly that at equitoxic doses of an analogue and calcitriol, the analogue has antitumor activity superior to calcitriol; nor have there been preclinical studies of any analogue which show that induction of hypercalcemia is less than that of calcitriol when given at “equi-effective” doses. The apparent reduction of hypercalcemia in preclinical models may be explained by differences in protein binding and catabolism of analogues compared to parent compound. Demonstrating that the dose of an analogue which causes hypercalcemia is larger than the dose of calcitriol that causes hypercalcemia does not establish that an analogue is “molecularly less hypercalcemic”. Ma et al. demonstrated that inecalcitol and calcitriol have different maximum tolerable doses in mice and that in vitro antitumor effects of inecalcitol were seen at lower concentrations of this agent than calcitriol. However, in a xenograft model of squamous cell carcinoma, doses of these two compounds that caused similar degrees of hypercalcemia also had similar antitumor effects (Ma et al., 2013). No vitamin D analogue has been developed which clearly dissociates the hypercalcemic effects of the agent from the anticancer effects.
7. Combination therapies with vitamin D compounds
Although calcitriol and analogues have interesting antitumor effects in preclinical models, single agents usually have limited effect in clinical cancer therapy; therefore, calcitriol combination therapies have been widely evaluated.
7.1. Glucocorticoids
Glucocorticoids have direct anticancer effects and block calcitriol-induced hypercalcemia. In addition, glucocorticoids enhance VDR expression in many cell types. Synergistic antitumor effects of calcitriol and glucocorticoids have been demonstrated in human prostate cancer xenografts, and the dexamethasone does reduce calcitriol-induced hypercalcemia in patients (Mccarthy et al., 1983; Trump et al., 2004).
7.2. Inhibitors of CYP24A1
Non-specific (e.g. ketoconazole and liarazole, isoflavones such as genistein, progesterone) and specific inhibitors of CYP24A1 each accentuate the antitumor activity of calcitriol (Trump et al., 2004; Zhang et al., 2012; Peehl et al., 2002; Swami et al., 2005; Muindi et al., 2010; Chiellini et al., 2012; Lechner et al., 2007; Yee & Simons, 2004; Schuster et al., 2003; Ly et al., 1999; Zhao et al., 1996; Rao et al., 2002; Rodriguez et al., 2016; Lee et al., 2013; Lou & Tuohimaa, 2006; Yee et al., 2006).
7.3. Non-steroidal anti-inflammatory drugs
Prostaglandin synthesis inhibitors may potentiate the activity of vitamin D compounds. A phase II trial of the nonselective inhibitor naproxen and high-dose calcitriol in patients with early recurrent prostate cancer demonstrated apparent activity as evidenced by reduction in PSA doubling time (Srinivas & Feldman, 2009).
7.4. Retinoids
Since calcitriol, VDR, RXR and 9-cisretinoic acid, interact in vitamin D signaling it is plausible to hypothesize that ligands for the retinoid receptors, RARs and RXRs might modify calcitriol activity. Potentiation of calcitriol antitumor effects by retinoid compounds has been well described (Gocek et al., 2012; Mernitz et al., 2007; Mouratidis et al., 2006; Peehl & Feldman, 2004; Ikeda et al., 2003; Elstner et al., 1999).
7.5. Cytotoxic agents
Both in vitro and in vivo, vitamin D compounds can be shown to potentiate the cytotoxicity of many anticancer agents. Calcitriol or its analogues potentiate platinum compounds (cisplatin [Platinol®], carboplatin [Paraplatin®], anthracyclines (doxorubicin [Adriamycin®], mitoxantrone [Novantrone®], topoisomerase Inhibitors (irinotecan [Camptosar®], etoposide [Etoposide®], antimetabolites (cytosine arabinoside [Cytarabine®], gemcitabine [Gemzar®], 5-fluorouracil [Adrucil®]) and taxanes (docetaxel [Docetaxel®] and paclitaxel [Taxol®]. The precise mechanism(s) of this potentiation are not clear (Smith et al., 1999; Johnson et al., 2002; Muindi et al., 2002; Muindi et al., 2005; Muindi et al., 2009; Fakih et al., 2007; Chan et al., 2008; Muindi et al., 2004). These effects are most pronounced when vitamin D compounds are administered before or simultaneously with the cytotoxic agent.
7.6. Ionizing radiation and photodynamic therapy
Vitamin D compounds potentiate the antitumor effects of ionizing radiation and photodynamic therapy though few clinical studies have been conducted with this approach (Dunlap et al., 2003; Anand et al., 2014).
8. Clinical evaluation of vitamin D compounds in cancer therapy
Many clinical trials have been conducted which have sought to define evidence of clinical benefit of calcitriol and analogues in the treatment of cancer patients (Osborn et al., 1995; Chadha et al., 2010; Trump et al., 2006; Morris et al., 2004; Schwartz et al., 2005; Gross et al., 1998; Beer et al., 2004a; Beer et al., 2003a; Liu et al., 2003; Beer et al., 2001). Suffice it to say, none of these trials has provided strong evidence of benefit or activity. With the benefit of hindsight, several factors have contributed to the failure to demonstrate beneficial effects of calcitriol or analogue therapy in most trials:
-
•
The majority of clinical trials of vitamin D compounds in cancer have been single institution, investigator-initiated trials. Coincidently, because several investigative teams have had interest in prostate cancer, a disproportionate amount of work has been done in men with prostate cancer. While single institution, investigator-initiated trials are not intrinsically flawed, they do tend to be underpowered, involving a relatively small number of patients; in addition, these trials have often tested combinations of calcitriol and other drugs with established antitumor activity (e.g. mitoxantrone, carboplatin, paclitaxel, docetaxel, dexamethasone, cisplatin, ketoconazole) in a given cancer; this confounds assessment of antitumor activity, particularly in small trials
-
•
The basic principles of anticancer drug development have been incompletely applied in studies of calcitriol and analogues. There has never been clear agreement on the best dose and schedule of calcitriol and analogues. Preclinical studies provide strong evidence for a substantial effect of exposure to calcitriol as a determinant of anticancer activity, especially when combined with cytotoxic agents. Nonetheless many studies have been done with particular attention to estimates of optimal dose or schedule of vitamin D compound.
-
•
An important factor that has hampered the development of vitamin D analogues as cancer therapies has been the lack of interest and commitment to this concept by any pharmaceutical company.
-
•
Absence of patent protection for other than new formulations of calcitriol has been a serious challenge. This is particularly ironic in that the most extensive data on the preclinical effects of vitamin D compounds in cancer models have been developed for calcitriol
-
•
There has been limited exploration of the activity of vitamin D analogues in cancer (e.g. paricalcitol, EB1089). Commitment to develop these analogues against cancer seems to have been limited by reluctance to commit to a cancer strategy for agents whose primary developmental path has been for chronic renal disease (paricalcitol) or general limitations on resources and interest (EB1089).
Dose and schedule are critical considerations when developing any new therapeutic. A series of studies examining daily oral, subcutaneous QOD and oral QDX3 weekly clearly have shown that the amount of calcitriol that can be administered safely is greatly enhanced by intermittent therapy (see below). However, many clinical studies were done using continuous therapy of every other day dosing – perhaps because vitamin D is a hormone? All studies of daily or QOD therapy have been negative. Preclinical studies of 1,25(OH)2D3 and analogues clearly demonstrate the following principles:
-
•
The anticancer activity of 1,25(OH)2D3 is clearly dose dependent. Based on this concept, clinical studies should be conducting utilizing the maximum safe dose of vitamin D compound.
-
•
Maximum exposure to 1,25(OH)2D3 compounds in animal studies is achieved if these agents are administered on an intermittent schedule.
One would expect these considerations would have been of clinical trials of vitamin D compounds in cancer therapy. As we will see, this has often not been the case.
9. Vitamin D analogues administered before prostatectomy: exploration of biologic effects
There is evidence that vitamin D compounds have biologic effects on human tissues. Several studies evaluated the effect of the administration of vitamin D compounds in the preoperative period before prostatectomy. This study design allows the exploration of clinically relevant dose and biologic response relationships in human tissues. In addition, such data provide evidence that complements preclinical work indicating changes in potentially important biomarkers of biologic effect of vitamin D in human cancer tissues in vivo. Beer and colleagues gave 39 patients either calcitriol weekly (0.5 μg/kg weekly × 4) or placebo and for 4 weeks prior to prostatectomy (Beer et al., 2004b). In the calcitriol group the percentage of cells expressing VDR was lower (75.3%) compared to the patients receiving placebo (98.6%), there was no change in the percentage of cells expressing TGFbeta RII, PTEN, or proliferating cell nuclear antigen (PCNA) (Beer et al., 2004b). Gee and colleagues studied patients randomized to daily doxercalciferol (10 μg QD × 28 days) versus placebo for 28 days prior to radical prostatectomy (Gee et al., 2013). Serum markers examined included vitamin D metabolites, TGFβ 1/2, free/total PSA, IGF-1, IGFBP-3, bFGF, and VEGF; tissue markers studied included histology, MIB-1 and TUNEL staining, microvessel density and factor VIII, staining, androgen receptor and PSA, VDR expression and nuclear morphometry. TGF-β2 was the only biomarker significantly altered by doxercalciferol supplementation (~2–4 fold). Wagner and colleagues evaluated the biologic effect of vitamin D3 (cholecalciferol) administration prior to prostatectomy on intraprostatic concentrations of vitamin D metabolites as well as on markers of potential biologic importance (Wagner et al., 2013). 63 patients received either 400 IU, 10,000 IU, or 40,000 IU daily by mouth for up to 4 weeks prior to prostatectomy. In prostate tissue, vitamin D metabolite levels and Ki67 labeling were assessed and serum PTH, and PSA were measured. This study demonstrated the safety of these dosing regimens and showed clearly that tissue and serum levels of vitamin D metabolites, including calcitriol, increased in a dose-dependent manner (P < .03). Vitamin D compound concentrations were highest in the 40,000-IU/d group (P < .03) and tissue vitamin D metabolite levels were correlated with vitamin D serum levels (P < .0001). While Ki67 labeling indices were not different among the dosing groups, there was evidence that intraprostatic calcitriol level was inversely associated with the proliferation marker, Ki67 intensity and percent Ki67 “positive” nuclei in prostate cancer and benign tissue (P < .05). The 2 highest dose supplementation groups were combined in an analysis of the impact of dosing on PTH and PSA serum levels which indicated that high dose D3 administration suppressed PTH and PSA levels. (P < .02). This group conducted further exploratory analyses on the tissues available from this clinical trial. VDR was detected in both epithelial and stromal tissues and vitamin D hydroxylases were present only in prostate stromal cells. VDR expression was suppressed in those tissues with the highest 1,25(OH)2D3 content. In specimens with the highest 1,25(OH)2D3 content, epithelial cell IL-6 was highest and stromal cell COX-2 was lowest (Giangreco et al., 2015). These studies in which either calcitriol, doxercalciferol or D3 were administered provided limited evidence of a strong biologic effect, effects were seen – following doses and schedules of calcitriol or doxercalciferol that were not in any way optimized for biologic effect; the data of Wagner and colleagues are consistent with the conclusion that higher concentrations of vitamin D compounds influence the biology of prostate tissues.
Rather than cite each of the several studies that evaluated calcitriol and analogues as single agents and in combination with glucocorticoids, epidermal growth factor receptor antagonists and cytotoxic agents, suffice it to say that these studies may be summarized as follows (Osborn et al., 1995; Chadha et al., 2010; Trump et al., 2006; Morris et al., 2004; Schwartz et al., 2005; Gross et al., 1998; Beer et al., 2004a; Beer et al., 2003a; Liu et al., 2003):
-
•
High doses of calcitriol can be given safely to cancer patients
-
•
No unusual or unexpected toxicity has been seen
-
•
No potentiation of cytotoxic agent toxicity has been noted
-
•
The only effect of calcitriol and analogues that has been noted has been mild – moderate transient hypercalcemia.
While enticing suggestions of clinically important anticancer effects have been seen, the limitations of these studies, as noted above (small sample sizes, failure to use biologically optimal or maximally tolerable doses and use in combination with other active agents) no definitive evidence of benefit has been documented.
Before discussing in detail two randomized controlled trials that had the potential to define, clearly, at least in men with castration resistant prostate cancer, the role of calcitriol and cytotoxic chemotherapy, let us consider three characteristics of calcitriol-based anticancer therapy that were confounding to its development:
9.1. Schedule
As noted above, considerable resources were invested in studies of calcitriol and analogues, that were – if maximizing tumor cell exposure to calcitriol was the goal – not likely to be positive. It is clear that the maximum oral dose of calcitriol is 1.5–2.0 μg QD (calcitriol exposure = ~14 μg/2 weeks) (Osborn et al., 1995). In contrast, intravenous calcitriol at a dose of 74 μg weekly is safe and well-tolerated. (calcitriol exposure = 144 μg/2 weeks) (Chadha et al., 2010). 125 μg/week is safe if combined with dexamethasone (calcitriol exposure = 250 μg/2 weeks) (Muindi et al., 2009). Yet many studies of daily or every other day oral calcitriol and analogues have been done. All were negative.
9.2. Dose
A more precise measure of exposure would be drug level achieved in blood or tumor. Muindi and colleagues demonstrated that the systemic exposure to calcitriol achieved in murine models in which calcitriol is effective in suppressing cancer growth can be achieved in humans at high, but safe and tolerable oral and intravenous doses of calcitriol (Muindi et al., 2009; Muindi et al., 2004). As will be discussed below, the largest studies of calcitriol in men with prostate cancer did not use doses high enough to approach these “target exposures”.
9.3. Formulation
As studies of high dose intermittent therapy with calcitriol were conducted it became clear that there is not a suitable formulation of calcitriol for such schedules. Two groups demonstrated that the available oral formulation (Rocaltrol®) is inappropriate for high dose administration. Doses of Rocaltrol® as high as 40 μg QDX3 (daily for 3 successive days) were investigated. This schedule is inconvenient (requiring 80 tablets daily) and pharmaceutically flawed. At doses >15–20 μg QD of Rocaltrol® the expected linear relationship between dose administered and plasma levels of calcitriol achieved is lost, apparently due to impaired absorption. Use of the liquid formulation of calcitriol did not overcome this problem (Muindi et al., 2002; Muindi et al., 2005; Liu et al., 2003).
The now defunct pharmaceutical company Novocea developed a new formulation of calcitriol referred to as DN-101. This formulation was carefully studied and did provide a linear relationship between dose and systemic exposure over a wide dose range (Beer et al., 2005; Beer et al., 2007a). With this new formulation in hand, Novocea undertook the evaluation of the potential for calcitriol to enhance the microtubule disrupting agent, docetaxel (Taxotere®) in the treatment of men with castration resistant prostate cancer (CRPC).
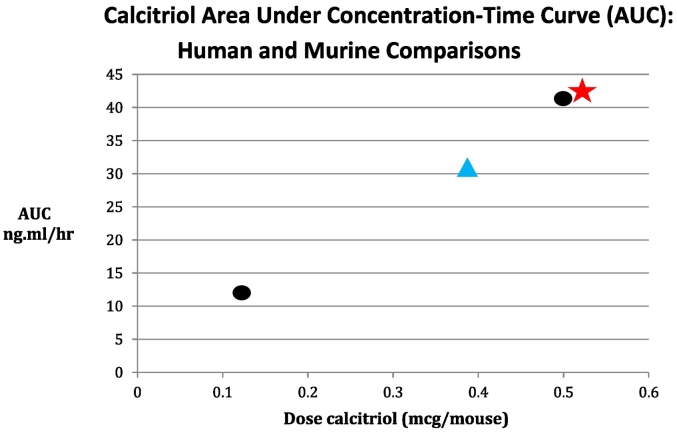
DN-101 had a favorable pharmaceutic profile: a linear relationship between dose and exposure over a wide dose range. At the 165 μg dose, C(max) was 6.21 ± 1.99 ng/mL, AUC(0–24) was 41.3 ± 9.77 ng ∗ h/mL, and half-life (T1/2) was 16.2 h. Muindi and colleagues noted that AUC and Cmax calcitriol concentrations of 32 ng ∗ h/mL and 9.2 ng/mL are associated with striking antitumor effects in a murine squamous cell carcinoma model (Fig. 1).
Fig. 1.
Calcitriol Area Under Concentration-Time Curve (AUC): human and murine comparisons.
Dose escalation in the initial phase 1 trial of single dose administration was halted at 165 μg orally × 1 (all human DN-101 development was done employing the oral route of administration) because of the inconvenience of higher doses which would have required >11 capsules to be administered. Might there have been advantage to reformulating the capsules into higher drug content and continuing dose escalation? In the subsequent repeated dosing (weekly, oral administration) phase 1 trial doses of 15, 30, 45, 60 and 75 μg were studied. Dose limiting toxicity (DLT) was defined as grade 2 or greater hypercalcemia or grade 3 or greater persistent treatment-related toxicities. The maximum tolerated dose (MTD) was defined as that dose level below which dose limiting toxicity occurred in 2 or more of 6 individuals at a given escalation level. At the 60 μg dose 2 of 6 patients experienced asymptomatic grade 2 hypercalcemia (serum calcium of 11.6–12.5 mg/dL). 45 μg weekly was defined as the MTD of DN-101 and 18 patients were treated with this dose without any dose limiting toxicities. This dose of 45 μg weekly was chosen for further development in combination with docetaxel (36 mg/m2, weekly) in CRPC define. Calcitriol had been combined with docetaxel in a phase II trial reported by Beer and colleagues. Prostate-specific antigen (PSA) decline of 50% or greater was seen in 81% of patients in that trial. The calcitriol dose in that study was 0.5 μg/kg or ~35 μg for a 70 kg man – very much in the range of the 45 μg weekly dose determined to be the MTD dose of DN-101. The 45 μg dose was chosen for further development. The decision as to the definition of dose-limiting toxicity (a single grade 2 elevation in serum calcium without regard to duration of elevation or biologic effects of that elevation) is exceedingly conservative. One could imagine this decision was influenced by the trial of Beer et al. demonstrating a high PSA response rate with a similar calcitriol dose – in a single institution, trial of 37 patients (Beer et al., 2003b). The extent of toxicity defined as dose limiting (grade 2 hypercalcemia) in the development of DN-101 is substantially less than that usually accepted in studies developing anticancer agents. In 2011 Le Tourneau and colleagues analyzed 155 phase I trials and found that “hypercalcemia” was not specifically noted to have been a DLT in any study, though electrolyte abnormalities were the defining DLT in <5% of trials. Overall, the great majority of studies employed greater than or equal to grade 3 toxicity as a DLT definition, supporting the observation that the MTD and DLT delineation for DN-101 was conservative (Eaton et al., 2016; Le Tourneau et al., 2011). In a trial of weekly intravenous calcitriol + gefitinib and fixed dose dexamethasone, Muindi and colleagues defined DLT as any of the following toxicities attributable to study treatment in the first 4 weeks of treatment: ≥3 or 4 toxicity except for grade 3 anemia, ≥grade 2 or above hypercalcemia (corrected calcium > 11.5 mg/dL) if confirmed on repeat blood draw (>72 h), any grade 2 or above hypercalcemia that is associated with serious hypercalcemic symptoms, any treatment interruption that lasts for >2 weeks that is related to treatment toxicity, sustained increase (>72 h) in creatinine to >2× baseline and >2 mg/dL, and clinical or radiological evidence of new genitourinary stones. With this definition among 20 patients dose-limiting hypercalcemia was observed in two out of the four patients receiving 163 μg/week of calcitriol. MTD was defined as 125 μg weekly and at this dose mean (±SE) peak serum calcitriol concentration (Cmax)was 11.17 ± 2.62 ng/mL and the systemic exposure (AUC0–72h) was 53.30 ± 10.49 ng h/mL (Muindi et al., 2009). Ramnath and colleagues studied escalating doses of intravenous calcitriol administered every 21 days prior to docetaxel 75 mg/m2 and cisplatin 75 mg/m2 in a phase I/II study. In the phase I portion of this trial intravenous calcitriol doses of 30, 45, 60, and 80 μg/m2 were studied. 14 patients were studied in phase I. At 80 μg/m2 2/4 patients had DLTs of grade 4 neutropenia. Hypercalcemia was not observed. The MTD was determined to be 60 μg/m2. Among 20 evaluable phase II patients, there were 6 partial responses (30%), and 9 patients with stable disease (45%). The role of calcitriol in the myelosuppression seen in this trial is unclear; no hypercalcemia was noted (Ramnath et al., 2013). These data support the hypothesis that the dose of DN-101 chosen for further development was very conservative.
Novocea conducted two randomized phase trials in men with CRPC: ASCENT I and ASCENT II (Beer et al., 2007b; Scher et al., 2011). The goal of ASCENT I was to determine the PSA response rate (defined as a >50% decline in PSA for >1 month) following standard therapy for CRPC (docetaxel 36 mg/m2 weekly intravenously × 4 weeks every 6 weeks) compared to the same dose and schedule of docetaxel + calcitriol (DN-101), 45 μg weekly. Was DN-101 iv or oral? Two hundred fifty patients were randomized. PSA response rates were 63% (DN-101) and 52% (placebo), (P-value = .07). While the primary goal (superior PSA response for DN-101 + docetaxel) of this trial was not achieved, there was a difference in the PSA response rate favoring the DN-101 arm. Survival was not a primary endpoint of this trial, but patients receiving DN-101 did fare better. Median survival for the placebo arm was 16.4 months and for the DN-101 arm 24.5 months. Hazard rate for death in the DN-101 group was 0.67 (p = .04). All toxicities appeared equal between the two arms and no patient experienced hypercalcemia. These results were encouraging, especially in view of the fact that this was a relatively large, randomized trial. Encouragement was derived primarily from a secondary endpoint analysis. Drug approval was not possible based on this trial. A larger trial was initiated (ASCENT II) in order to evaluate the apparent survival advantage of DN-101 + docetaxel.
In the time period between the design of ASCENT trials I and II, the weekly docetaxel regimen employed in ASCENT I clearly was shown to be inferior in terms of patient survival compared to an every three week regimen (75 mg/m2 every three weeks) (Petrylak et al., 2004; Tannock et al., 2004). Petrylak and colleagues reported a trial which compared docetaxel (weekly)/estramustine to mitoxantrone/prednisone in 770 patients with CRPC, and found an improvement in overall survival in the docetaxel arm (median survival 17.5 months for weekly docetaxel/estramustine vs. 15.6 months). Tannock and colleagues reported the TAX327 trial which compared q 3 week docetaxel, weekly docetaxel, and q 3 week mitoxantrone, with prednisone in 1006 patients. The patients who received q3 week docetaxel had a median survival of 18.9 months (reduction in risk of death = 24%), compared to 17.3 months for weekly docetaxel and 16.4 months for the mitoxantrone arm. In the TAX327 trial the q3 week docetaxel arm had higher rates of toxicity than the other 2 arms, (neutropenia and neuro-sensory changes); quality of life data suggest that docetaxel (either weekly or q3 week) is better tolerated than mitoxantrone.
If the designers of the ASCENT trials had adhered strictly to the principles of clinical trial design this change in the standard of care should have been accompanied by a change in the design of ASCENT II. The optimal design would have been docetaxel, 75 mg/m2 q3weeks ± DN-101. However, the ASCENT II trial designers decided to compare the apparently active, experimental treatment arm of ASCENT I (docetaxel, weekly, 36 mg/m2 + DN-101, 45 μg weekly × 4, every 6 weeks) to docetaxel 75 mg/m2 every three weeks + placebo. The enrollment target was 1200 patients. Complete the sentence. The final enrollment target was based on the assumption of a median survival of 18.9 months in the control group (q3weekly docetaxel + placebo) and a hazard ratio for mortality of ≤0.78 using a two-sided significance level of 0.05, with a power of 90%. This design tested the hypothesis that calcitriol would make an inferior treatment (weekly docetaxel) superior to a statistically better treatment (every 3 week docetaxel). The reasons for the decision to proceed with this “non-standard” trial design may have included:
-
(1)
Cost – expense would have been required to be sure q3week docetaxel, 75 mg/m2 + 45 μg DN-101 was safe and active.
-
(2)
Time – since the duration of patent protection on a composition of matter patent such as that which protected DN-101, is limited, time invested in resetting the experimental arm would have been a business risk
-
(3)
“Conviction” that calcitriol was sufficiently active in enhancing docetaxel in CRPC may have played a role.
ASCENT II was initiated on February 9, 2006. On October 31, 2007, when 953 patients had been accrued, the data and safety monitoring board recommended the study be halted. The reason for study interruption was a greater number of deaths in the experimental arm (weekly X4 docetaxel, q6weeks + weekly DN-101) than the control arm (q3weekly docetaxel + placebo). In the experimental (DN-101) arm median overall survival was 17.8 months (95% CI, 16.0 to 19.5) and 20.2 months (95% CI, 18.8 to 23.0) in the docetaxel only arm (log-rank P = .002). This trial result has been interpreted to show that calcitriol does not potentiate the antitumor efficacy of docetaxel in CRPC. And, largely, that further studies of vitamin D as a therapeutic agent in cancer were unwarranted. On reflection, ASCENT II could be described more accurately as demonstrating that a calcitriol dose less than that which can be safely administered does not overcome the inferiority of weekly vs. q3 weekly docetaxel. This trial was seriously flawed in two ways:
-
•
The design of ASCENT II violated a basic principle of clinical trial development. The appropriate standard for trial design is standard therapy ± investigational regimen. The scientific conclusion from ASCENT II is that calcitriol at the dose studied was not sufficiently potent to overcome the intrinsic inferiority of the weekly docetaxel regimen.
-
•
There are no data that indicate that the dose of calcitriol administered in ASCENT II was biologically or therapeutically ideal. The dose studied was <50% of the maximum tolerated dose of calcitriol that can be given on an intermittent schedule. The dose of calcitriol chosen was based on a 37 patient study by Beer and colleagues which demonstrated an 81% response rate in CRPC and the phase 1 study of DN-101 which employed a very conservative definition of MTD.
Ramnath et al. safely administered 80 μg/m2 (~120 μg total dose) of calcitriol intravenously with docetaxel and cisplatin and no patient had hypercalcemia (Ramnath et al., 2013). Myelosuppression was the limiting toxicity in that trial, likely related solely to the cytotoxic agents. Muindi et al. and Fakih et al. studied intravenous calcitriol weekly with gefitinib (Iressa®), an oral epidermal growth factor tyrosine kinase inhibitor. 74 μg weekly alone and 125 μg weekly with dexamethasone were the defined “phase II” doses (Muindi et al., 2009; Fakih et al., 2007). The ASCENT I and II trials were done with a dose of calcitriol that was one-quarter to one-half the calcitriol dose that would have been safe. Considerable evidence in preclinical studies indicates that the anticancer effect of calcitriol is dose/exposure related. Aside from the substantial number of studies noted above in prostate cancer and lung cancer, very limited studies have been done with calcitriol or its analogues in other cancers. Each of those studies utilized seocalcitol (EB1089) in breast, colorectal, pancreatic and hepatocellular carcinomas and is limited by concerns noted previously: (1) small sample size (2) much less than the MTD of analogue administered (3) use of continuous rather than intermittent dosing regimens (Limaye et al., 2013; Dalhoff et al., 2003; Evans et al., 2002; Gulliford et al., 1998).
10. Summary
There are considerable data indicating the importance of vitamin D signaling in cancer. Vitamin D signaling is a plausible target for the treatment of established cancers – either as vitamin D agents alone or such agents combined with other antineoplastic agents. Unfortunately, there still is limited information regarding the role of vitamin D compounds in the treatment of cancer. Two approaches could be considered to refine the clinical studies of vitamin D in prostate cancer:
-
(1)
Establish dependable biomarkers predictive of vitamin D response.
This could facilitate:
-
•
Selection of biologically appropriate or active dose of vitamin D compounds to study therapeutically
-
•
Selection of patients with a greater likelihood of response. This is the routinely employed approach that has led to the success in developing many so-called “targeted” cancer therapies (Solomon et al., 2014; Drilon et al., 2018; Limaye et al., 2013).
-
(2)
Conduct of well-designed, appropriately powered clinical trials of biologically appropriate doses of vitamin D compounds.
Existing data strongly support the continued development of these approaches.
Transparency document
Transparency document
Footnotes
The Transparency document associated with this article can be found, in online version.
References
- Abe E., Miyaura C., Sakagami H., Takeda M., Konno K., Yamazaki T., Yoshiki S., Suda T. Differentiation of mouse myeloid leukemia cells induced by 1 alpha,25-dihydroxyvitamin D3. Proc. Natl. Acad. Sci. U. S. A. 1981;78:4990–4994. doi: 10.1073/pnas.78.8.4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe J., Moriya Y., Saito M., Sugawara Y., Suda T., Nishii Y. Modulation of cell growth, differentiation, and production of interleukin-3 by 1 alpha,25-dihydroxyvitamin D3 in the murine myelomonocytic leukemia cell line WEHI-3. Cancer Res. 1986;46:6316–6321. [PubMed] [Google Scholar]
- Afzal S., Bojesen S.E., Nordestgaard B.G. Low plasma 25-hydroxyvitamin D and risk of tobacco-related cancer. Clin. Chem. 2013;59:771–780. doi: 10.1373/clinchem.2012.201939. [DOI] [PubMed] [Google Scholar]
- Alagbala A.A., Johnson C.S., Trump D.L., Posner G.H., Foster B.A. Antitumor effects of two less-calcemic vitamin D analogs (Paricalcitol and QW-1624F2-2) in squamous cell carcinoma cells. Oncology. 2006;70:483–492. doi: 10.1159/000098813. [DOI] [PubMed] [Google Scholar]
- Anand S., Rollakanti K.R., Horst R.L., Hasan T., Maytin E.V. Combination of oral vitamin D3 with photodynamic therapy enhances tumor cell death in a murine model of cutaneous squamous cell carcinoma. Photochem. Photobiol. 2014;90:1126–1135. doi: 10.1111/php.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aparna R., Subhashini J., Roy K.R., Reddy G.S., Robinson M., Uskokovic M.R. Selective inhibition of cyclooxygenase-2 (COX-2) by 1alpha,25-dihydroxy-16-ene-23-yne-vitamin D3, a less calcemic vitamin D analog. J. Cell. Biochem. 2008;104:1832–1842. doi: 10.1002/jcb.21749. [DOI] [PubMed] [Google Scholar]
- Basudhar D., Glynn S.A., Greer M., Somasundaram V., No J.H., Scheiblin D.A., Garrido P., Heinz W.F., Ryan A.E., Weiss J.M., Cheng R.Y.S., Ridnour L.A., Lockett S.J., McVicar D.W., Ambs S., Wink D.A. Coexpression of NOS2 and COX2 accelerates tumor growth and reduces survival in estrogen receptor-negative breast cancer. Proc. Natl. Acad. Sci. U. S. A. 2017;114:13030–13035. doi: 10.1073/pnas.1709119114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer S.R., Hankinson S.E., Bertone-Johnson E.R., Ding E.L. Plasma vitamin D levels, menopause, and risk of breast cancer: dose-response meta-analysis of prospective studies. Medicine (Baltimore) 2013;92:123–131. doi: 10.1097/MD.0b013e3182943bc2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer T.M., Munar M., Henner W.D. A phase I trial of pulse calcitriol in patients with refractory malignancies: pulse dosing permits substantial dose escalation. Cancer. 2001;91:2431–2439. [PubMed] [Google Scholar]
- Beer T.M., Lemmon D., Lowe B.A., Henner W.D. High-dose weekly oral calcitriol in patients with a rising PSA after prostatectomy or radiation for prostate carcinoma. Cancer. 2003;97:1217–1224. doi: 10.1002/cncr.11179. [DOI] [PubMed] [Google Scholar]
- Beer T.M., Eilers K.M., Garzotto M., Egorin M.J., Lowe B.A., Henner W.D. Weekly high-dose calcitriol and docetaxel in metastatic androgen-independent prostate cancer. J. Clin. Oncol. 2003;21:123–128. doi: 10.1200/jco.2003.05.117. [DOI] [PubMed] [Google Scholar]
- Beer T.M., Garzotto M., Katovic N.M. High-dose calcitriol and carboplatin in metastatic androgen-independent prostate cancer. Am. J. Clin. Oncol. 2004;27:535–541. doi: 10.1097/01.coc.0000136020.27904.9c. [DOI] [PubMed] [Google Scholar]
- Beer T.M., Myrthue A., Garzotto M., O'Hara M.F., Chin R., Lowe B.A. Randomized study of high-dose pulse calcitriol or placebo prior to radical prostatectomy. Cancer Epidemiol. Biomark. Prev. 2004;13:2225–2232. [PubMed] [Google Scholar]
- Beer T.M., Javle M., Lam G.N., Henner W.D., Wong A., Trump D.L. Pharmacokinetics and tolerability of a single dose of DN-101, a new formulation of calcitriol, in patients with cancer. Clin. Cancer Res. 2005;11(21):7794–7799. doi: 10.1158/1078-0432.CCR-05-0552. [DOI] [PubMed] [Google Scholar]
- Beer T.M., Javle M.M., Ryan C.W., Garzotto M., Lam G.N., Wong A. Phase I study of weekly DN-101, a new formulation of calcitriol, in patients with cancer. Cancer Chemother. Pharmacol. 2007;59:581–587. doi: 10.1007/s00280-006-0299-1. [DOI] [PubMed] [Google Scholar]
- Beer T.M., Ryan C.W., Venner P.M., Petrylak D.P., Chatta G.S., Ruether J.D. Double-blinded randomized study of high-dose calcitriol plus docetaxel compared with placebo plus docetaxel in androgen-independent prostate cancer: a report from the ASCENT Investigators. J. Clin. Oncol. 2007;25:669–674. doi: 10.1200/JCO.2006.06.8197. [DOI] [PubMed] [Google Scholar]
- Berkovich L., Sintov A.C., Ben-Shabat S. Inhibition of cancer growth and induction of apoptosis by BGP-13 and BGP-15, new calcipotriene-derived vitamin D3 analogs, in-vitro and in-vivo studies. Investig. New Drugs. 2013;31:247–255. doi: 10.1007/s10637-012-9839-1. [DOI] [PubMed] [Google Scholar]
- Bernardi R.J., Trump D.L., Yu W.D., McGuire T.F., Hershberger P.A., Johnson C.S. Combination of 1alpha,25-dihydroxyvitamin D(3) with dexamethasone enhances cell cycle arrest and apoptosis: role of nuclear receptor cross-talk and Erk/Akt signaling. Clin. Cancer Res. 2001;7:4164–4173. [PubMed] [Google Scholar]
- Binderup L., Latini S., Binderup E., Bretting C., Calverley M., Hansen K. 20-epi-vitamin D3 analogues: a novel class of potent regulators of cell growth and immune responses. Biochem. Pharmacol. 1991;42:1569–1575. doi: 10.1016/0006-2952(91)90426-6. [DOI] [PubMed] [Google Scholar]
- Bittenbring J.T., Neumann F., Altmann B., Achenbach M., Reichrath J., Ziepert M., Geisel J., Regitz E., Held G., Pfreundschuh M. Vitamin D deficiency impairs rituximab-mediated cellular cytotoxicity and outcome of patients with diffuse large B-cell lymphoma treated with but not without rituximab. J. Clin. Oncol. 2014;32:3242–3248. doi: 10.1200/JCO.2013.53.4537. [DOI] [PubMed] [Google Scholar]
- Byun D.J., Wolchok J.D., Rosenberg L.M., Girotra M. Cancer immunotherapy - immune checkpoint blockade and associated endocrinopathies. Nat. Rev. Endocrinol. 2017;13:195–207. doi: 10.1038/nrendo.2016.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M.J., Reddy G.S., Koeffler H.P. Vitamin D3 analogs and their 24-oxo metabolites equally inhibit clonal proliferation of a variety of cancer cells but have differing molecular effects. J. Cell. Biochem. 1997;66:413–425. [PubMed] [Google Scholar]
- Chadha M.K., Tian L., Mashtare T., Payne V., Silliman C., Levine E. Phase 2 trial of weekly intravenous 1,25 dihydroxy cholecalciferol (calcitriol) in combination with dexamethasone for castration-resistant prostate cancer. Cancer. 2010;116:2132–2139. doi: 10.1002/cncr.24973. [DOI] [PubMed] [Google Scholar]
- Chan J.S., Beer T.M., Quinn D.I., Pinski J.K., Garzotto M., Sokoloff M. A phase II study of high-dose calcitriol combined with mitoxantrone and prednisone for androgen-independent prostate cancer. BJU Int. 2008;102:1601–1606. doi: 10.1111/j.1464-410X.2008.08017.x. [DOI] [PubMed] [Google Scholar]
- Chen S., Zhu J., Zuo S., Ma J., Zhang J., Chen G. 1,25(OH)2D3 attenuates TGF-beta1/beta2-induced increased migration and invasion via inhibiting epithelial-mesenchymal transition in colon cancer cells. Biochem. Biophys. Res. Commun. 2015;468:130–135. doi: 10.1016/j.bbrc.2015.10.146. [DOI] [PubMed] [Google Scholar]
- Chen P.T., Hsieh C.C., Wu C.T., Yen T.C., Lin P.Y., Chen W.C. 1alpha,25-Dihydroxyvitamin D3 inhibits esophageal squamous cell carcinoma progression by reducing IL6 signaling. Mol. Cancer Ther. 2015;14:1365–1375. doi: 10.1158/1535-7163.MCT-14-0952. [DOI] [PubMed] [Google Scholar]
- Chiellini G., Rapposelli S., Zhu J., Massarelli I., Saraceno M., Bianucci A.M. Synthesis and biological activities of vitamin D-like inhibitors of CYP24 hydroxylase. Steroids. 2012;77:212–223. doi: 10.1016/j.steroids.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung I., Han G., Seshadri M., Gillard B.M., Yu W.D., Foster B.A. Role of vitamin D receptor in the antiproliferative effects of calcitriol in tumor-derived endothelial cells and tumor angiogenesis in vivo. Cancer Res. 2009;69:967–975. doi: 10.1158/0008-5472.CAN-08-2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colston K.W., Hansen C.M. Mechanisms implicated in the growth regulatory effects of vitamin D in breast cancer. Endocr. Relat. Cancer. 2002;9:45–59. doi: 10.1677/erc.0.0090045. [DOI] [PubMed] [Google Scholar]
- Colston K., Colston M.J., Feldman D. 1,25-dihydroxyvitamin D3 and malignant melanoma: the presence of receptors and inhibition of cell growth in culture. Endocrinology. 1981;108:1083–1086. doi: 10.1210/endo-108-3-1083. [DOI] [PubMed] [Google Scholar]
- Colston K., Colston M.J., Fieldsteel A.H., Feldman D. 1,25-dihydroxyvitamin D3 receptors in human epithelial cancer cell lines. Cancer Res. 1982;42:856–859. [PubMed] [Google Scholar]
- de Cremoux P., Hamy A.S., Lehmann-Che J., Scott V., Sigal B., Mathieu M.C., Bertheau P., Guinebretière J.M., Pierga J.Y., Giacchetti S., Brain E., Marty M., Asselain B., Spyratos F., Bièche I. COX2/PTGS2 expression is predictive of response to neoadjuvant celecoxib in HER2-negative breast Cancer patients. Anticancer Res. 2018;38:1485–1490. doi: 10.21873/anticanres.12375. [DOI] [PubMed] [Google Scholar]
- Dalhoff K., Dancey J., Astrup L., Skovsgaard T., Hamberg K.J., Lofts F.J., Rosmorduc O., Erlinger S., Bach Hansen J., Steward W.P., Skov T., Burcharth F., Evans T.R. A phase II study of the vitamin D analogue seocalcitol in patients with inoperable hepatocellular carcinoma. Br. J. Cancer. 2003;21(89):252–257. doi: 10.1038/sj.bjc.6601104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davila-Gonzalez D., Chang J.C., Billiar T.R. NO and COX2: dual targeting for aggressive cancers. Proc. Natl. Acad. Sci. U. S. A. 2017;114:13591–13593. doi: 10.1073/pnas.1717440114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty D., Dvorkin S.A., Rodriguez E.P., Thompson P.D. Vitamin D receptor agonist EB1089 is a potent regulator of prostatic “intracrine” metabolism. Prostate. 2014;74:273–285. doi: 10.1002/pros.22748. [DOI] [PubMed] [Google Scholar]
- Drilon A., Laetsch T., Kummar S., DuBois S., Lassen U., Demetri G., Nathenson M., Doebele R., Farago A., Pappo A., Turpin B., Dowlati A., Brose M., Mascarenhas L., Federman N., Berlin J., El-Deiry W., Baik C., Deeken J., Boni V., Nagasubramanian R., Taylor M., Rudzinski E., Meric-Bernstam F., Sohal D., Ma P., Raez L., Hechtman J., Benayed R., Ladanyi M., Tuch B., Ebata K., Cruickshank S., Ku N., Cox M., Hawkins D., Hong D., Hyman D. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N. Engl. J. Med. 2018;378:731–739. doi: 10.1056/NEJMoa1714448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy M.J., Murray A., Synnott N.C., O'Donovan N., Crown J. Vitamin D analogues: potential use in cancer treatment. Crit. Rev. Oncol. Hematol. 2017;112:190–197. doi: 10.1016/j.critrevonc.2017.02.015. [DOI] [PubMed] [Google Scholar]
- Dunlap N., Schwartz G.G., Eads D., Cramer S.D., Sherk A.B., John V. 1alpha,25-dihydroxyvitamin D(3) (calcitriol) and its analogue, 19-nor-1alpha,25(OH)(2)D(2), potentiate the effects of ionising radiation on human prostate cancer cells. Br. J. Cancer. 2003;89:746–753. doi: 10.1038/sj.bjc.6601161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton A., Iasonos A., Gounder Pamer M., Drilon A., Vulih D., Smith G., Ivy S., Spriggs D., Hyman D. Toxicity attribution in phase I trials: evaluating the effect of dose on the frequency of related and unrelated toxicities. Clin. Cancer Res. 2016;22:553–559. doi: 10.1158/1078-0432.CCR-15-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisman J.A., Martin T.J., MacIntyre I., Moseley J.M. 1,25-dihydroxyvitamin-D-receptor in breast cancer cells. Lancet. 1979;2:1335–1336. doi: 10.1016/s0140-6736(79)92816-2. [DOI] [PubMed] [Google Scholar]
- Eisman J.A., Macintyre I., Martin T.J., Frampton R.J., King R.J. Normal and malignant breast tissue is a target organ for 1,25-(0H)2 vitamin D3. Clin. Endocrinol. 1980;3:267–272. doi: 10.1111/j.1365-2265.1980.tb01053.x. [DOI] [PubMed] [Google Scholar]
- Eisman J.A., Martin T.J., MacIntyre I. 1,25-dihydroxyvitamin D3 receptors in cancer. Lancet. 1980;1:1188. doi: 10.1016/s0140-6736(80)91643-8. [DOI] [PubMed] [Google Scholar]
- Eisman J.A., Martin T.J., MacIntyre I. Presence of 1,25-dihydroxy vitamin D receptor in normal and abnormal breast tissue. Prog. Biochem. Pharmacol. 1980;17:143–150. [PubMed] [Google Scholar]
- Eisman J.A., Suva L.J., Sher E., Pearce P.J., Funder J.W., Martin T.J. Frequency of 1,25-dihydroxyvitamin D3 receptor in human breast cancer. Cancer Res. 1981;41:5121–5124. [PubMed] [Google Scholar]
- Eisman J.A., Frampton R.J., McLean F.J. Biochemical significance of enhanced activity of fluorinated 1,25-dihydroxyvitamin D3 in human cultured cell lines. Cell Biochem. Funct. 1986;4:115–122. doi: 10.1002/cbf.290040207. [DOI] [PubMed] [Google Scholar]
- Elstner E., Campbell M.J., Munker R., Shintaku P., Binderup L., Heber D. Novel 20-epi-vitamin D3 analog combined with 9-cis-retinoic acid markedly inhibits colony growth of prostate cancer cells. Prostate. 1999;40:141–149. doi: 10.1002/(sici)1097-0045(19990801)40:3<141::aid-pros1>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Evans T.R., Colston K.W., Lofts F.J., Cunningham D., Anthoney D.A., Gogas H., de Bono J.S., Hamberg K.J., Skov T., Mansi J.L. A phase II trial of the vitamin D analogue Seocalcitol (EB1089) in patients with inoperable pancreatic cancer. Br. J. Cancer. 2002;86:680–685. doi: 10.1038/sj.bjc.6600162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakih M.G., Trump D.L., Muindi J.R., Black J.D., Bernardi R.J., Creaven P.J. A phase I pharmacokinetic and pharmacodynamic study of intravenous calcitriol in combination with oral gefitinib in patients with advanced solid tumors. Clin. Cancer Res. 2007;13:1216–1223. doi: 10.1158/1078-0432.CCR-06-1165. [DOI] [PubMed] [Google Scholar]
- Feldman D., Krishnan A.V., Swami S., Giovannucci E., Feldman B.J. The role of vitamin D in reducing cancer risk and progression. Nat. Rev. Cancer. 2014;14:342–357. doi: 10.1038/nrc3691. [DOI] [PubMed] [Google Scholar]
- Feng Q., Zhang H., Dong Z., Zhou Y., Ma J. Circulating 25-hydroxyvitamin D and lung cancer risk and survival: a dose-response meta-analysis of prospective cohort studies. Medicine (Baltimore) 2017;96 doi: 10.1097/MD.0000000000008613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira G.B., Overbergh L., Verstuyf A., Mathieu C. 1alpha,25-Dihydroxyvitamin D3 and its analogs as modulators of human dendritic cells: a comparison dose-titration study. J. Steroid Biochem. Mol. Biol. 2013;136:160–165. doi: 10.1016/j.jsbmb.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Frampton R.J., Suva L.J., Eisman J.A., Findlay D.M., Moore G.E., Moseley J.M., Martin T.J. Presence of 1,25-dihydroxyvitamin D3 receptors in established human cancer cell lines in culture. Cancer Res. 1982;42:1116–1119. [PubMed] [Google Scholar]
- Frampton R.J., Omond S.A., Eisman J.A. Inhibition of human cancer cell growth by 1,25-dihydroxyvitamin D3 metabolites. Cancer Res. 1983;43(9):4443–4447. [PubMed] [Google Scholar]
- Frankel T.L., Mason R.S., Hersey P., Murray E., Posen S. The synthesis of vitamin D metabolites by human melanoma cells. J. Clin. Endocrinol. Metab. 1983;57:627–631. doi: 10.1210/jcem-57-3-627. [DOI] [PubMed] [Google Scholar]
- Freake H.C., MacIntyre I. Specific binding of 1,25-dihydroxycholecalciferol in human medullary thyroid carcinoma. Biochem. J. 1982;206:181–184. doi: 10.1042/bj2060181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freake H.C., Iwasaki J., McCarthy D.M. Specific uptake of 1,25-dihydroxycholecalciferol by human chronic myeloid leukemia cells. Cancer Res. 1984;44:3627–3631. [PubMed] [Google Scholar]
- Fry J.M., Curnow D.H., Retallack R.W., Gutteridge D.H. Significance of 1, 25-dihydroxyvitamin-D “receptors” in normal and malignant breast tissue. Lancet. 1980;1:1308–1309. doi: 10.1016/s0140-6736(80)91771-7. [DOI] [PubMed] [Google Scholar]
- Gandini S., Boniol M., Haukka J. Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int. J. Cancer. 2011;128:1414–1424. doi: 10.1002/ijc.25439. [DOI] [PubMed] [Google Scholar]
- Garland C.F., Gorham E.D. Dose-response of serum 25-hydroxyvitamin D in association with risk of colorectal cancer: a meta-analysis. J. Steroid Biochem. Mol. Biol. 2017;168:1–8. doi: 10.1016/j.jsbmb.2016.12.003. [DOI] [PubMed] [Google Scholar]
- Gee J., Bailey H., Kim K., Kolesar J., Havighurst T., Tutsch K.D. Phase II open label, multi-center clinical trial of modulation of intermediate endpoint biomarkers by 1alpha-hydroxyvitamin D2 in patients with clinically localized prostate cancer and high grade pin. Prostate. 2013;73:970–978. doi: 10.1002/pros.22644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giangreco A.A., Dambal S., Wagner D., Van der Kwast T., Vieth R., Prins G.S. Differential expression and regulation of vitamin D hydroxylases and inflammatory genes in prostate stroma and epithelium by 1,25-dihydroxyvitamin D in men with prostate cancer and an in vitro model. J. Steroid Biochem. Mol. Biol. 2015;148:156–165. doi: 10.1016/j.jsbmb.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gocek E., Marchwicka A., Baurska H., Chrobak A., Marcinkowska E. Opposite regulation of vitamin D receptor by ATRA in AML cells susceptible and resistant to vitamin D-induced differentiation. J. Steroid Biochem. Mol. Biol. 2012;132:220–226. doi: 10.1016/j.jsbmb.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Going C.C., Alexandrova L., Lau K., Yeh C.Y., Feldman D., Pitteri S.J. Vitamin D supplementation decreases serum 27-hydroxycholesterol in a pilot breast cancer trial. Breast Cancer Res. Treat. 2018;167:797–802. doi: 10.1007/s10549-017-4562-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Cao M., Karachaliou N., Viteri S., Morales-Espinosa D., Teixido C., Sanchez Ruiz J. Targeting PD-1/PD-L1 in lung cancer: current perspectives. Lung Cancer. 2015;6:55–70. doi: 10.2147/LCTT.S55176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Pardo V., Verstuyf A., Boland R., Russo de Boland A. Vitamin D analogue TX 527 down-regulates the NF-κB pathway and controls the proliferation of endothelial cells transformed by Kaposi sarcoma herpesvirus. Br. J. Pharmacol. 2013;169:1635–1645. doi: 10.1111/bph.12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Pardo V., Suares A., Verstuyf A., De Clercq P., Boland R., de Boland A.R. Cell cycle arrest and apoptosis induced by 1α,25(OH)2D3 and TX 527 in Kaposi sarcoma is VDR dependent. J. Steroid Biochem. Mol. Biol. 2014;144:197–200. doi: 10.1016/j.jsbmb.2013.11.014. [DOI] [PubMed] [Google Scholar]
- Grant W.B. Ecological studies of the UVB-vitamin D-cancer hypothesis. Anticancer Res. 2012;32:223–236. [PubMed] [Google Scholar]
- Gross C., Stamey T., Hancock S., Feldman D. Treatment of early recurrent prostate cancer with 1,25-dihydroxyvitamin D3 (calcitriol) J. Urol. 1998;159:2035–2039. doi: 10.1016/S0022-5347(01)63236-1. (discussion 9-40) [DOI] [PubMed] [Google Scholar]
- Gulliford T., English J., Colston K.W., Menday P., Moller S., Coombes R.C. A phase I study of the vitamin D analogue EB 1089 in patients with advanced breast and colorectal cancer. Br. J. Cancer. 1998;78:6–13. doi: 10.1038/bjc.1998.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Srivastava M., Ahmad N., Bostwick D.G., Mukhtar H. Over-expression of cyclooxygenase-2 in human prostate adenocarcinoma. Prostate. 2000;42:73–78. doi: 10.1002/(sici)1097-0045(20000101)42:1<73::aid-pros9>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Haq M., Kremer R., Goltzman D., Rabbani S.A. A vitamin D analogue (EB1089) inhibits parathyroid hormone-related peptide production and prevents the development of malignancy-associated hypercalcemia in vivo. J. Clin. Invest. 1993;91:2416–2422. doi: 10.1172/JCI116475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma Y., Hozumi M., Abe E., Konno K., Fukushima M., Hata S., Nishii Y., DeLuca H.F., Suda T. 1 alpha,25-Dihydroxyvitamin D3 and 1 alpha-hydroxyvitamin D3 prolong survival time of mice inoculated with myeloid leukemia cells. Proc. Natl. Acad. Sci. U. S. A. 1983;80:201–204. doi: 10.1073/pnas.80.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y.F., Gao S.H., Wang P., Zhang H.M., Liu L.Z., Ye M.X. 1alpha,25(OH)(2)D(3) suppresses the migration of ovarian cancer SKOV-3 cells through the inhibition of epithelial-mesenchymal transition. Int. J. Mol. Sci. 2016;17 doi: 10.3390/ijms17081285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda N., Uemura H., Ishiguro H., Hori M., Hosaka M., Kyo S. Combination treatment with 1alpha,25-dihydroxyvitamin D3 and 9-cis-retinoic acid directly inhibits human telomerase reverse transcriptase transcription in prostate cancer cells. Mol. Cancer Ther. 2003;2:739–746. [PubMed] [Google Scholar]
- Irani M., Seifer D.B., Grazi R.V., Julka N., Bhatt D., Kalgi B. Vitamin D supplementation decreases TGF-beta1 bioavailability in PCOS: a randomized placebo-controlled trial. J. Clin. Endocrinol. Metab. 2015;100:4307–4314. doi: 10.1210/jc.2015-2580. [DOI] [PubMed] [Google Scholar]
- Ishizaki F., Nishiyama T., Kawasaki T., Miyashiro Y., Hara N., Takizawa I., Naito M., Takahashi K. Androgen deprivation promotes intratumoral synthesis of dihydrotestosterone from androgen metabolites in prostate cancer. Sci. Rep. 2013;3:1528. doi: 10.1038/srep01528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James S.Y., Mackay A.G., Colston K.W. Effects of 1,25 dihydroxyvitamin D3 and its analogues on induction of apoptosis in breast cancer cells. J. Steroid Biochem. Mol. Biol. 1996;58:395–401. doi: 10.1016/0960-0760(96)00048-9. [DOI] [PubMed] [Google Scholar]
- JH1 Beumer, Parise R.A., Kanterewicz B., Petkovich M., D'Argenio D.Z., Hershberger P.A. A local effect of CYP24 inhibition on lung tumor xenograft exposure to 1,25-dihydroxyvitamin D(3) is revealed using a novel LC-MS/MS assay. Steroids. 2012;77:477–483. doi: 10.1016/j.steroids.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C.S., Hershberger P.A., Bernardi R.J., McGuire T.F., Trump D.L. Vitamin D receptor: a potential target for intervention. Urology. 2002;60:123–130. doi: 10.1016/s0090-4295(02)01591-1. (discussion 30-1) [DOI] [PubMed] [Google Scholar]
- Johnson C.S., Muindi J.R., Hershberger P.A., Trump D.L. The antitumor efficacy of calcitriol: preclinical studies. Anticancer Res. 2006;26:2543–2549. [PubMed] [Google Scholar]
- Kelly J.L., Salles G., Goldman B., Fisher R.I., Brice P., Press O., Casasnovas O., Maloney D.G., Soubeyran P., Rimsza L., Haioun C., Xerri L., LeBlanc M., Tilly H., Friedberg J.W. Low serum vitamin D levels are associated with inferior survival in follicular lymphoma: a prospective evaluation in SWOG and LYSA studies. J. Clin. Oncol. 2015;33:1482–1490. doi: 10.1200/JCO.2014.57.5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeffler H.P., Amatruda T., Ikekawa N., Kobayashi Y., DeLuca H.F. Induction of macrophage differentiation of human normal and leukemic myeloid stem cells by 1,25-dihydroxyvitamin D3 and its fluorinated analogues. Cancer Res. 1984;44:5624–5628. [PubMed] [Google Scholar]
- Koeffler H.P., Hirji K., Itri L. 1,25-Dihydroxyvitamin D3: in vivo and in vitro effects on human preleukemic and leukemic cells. Cancer Treat. Rep. 1985;69:1399–1407. [PubMed] [Google Scholar]
- Krishnan A.V., Feldman D. Molecular pathways mediating the anti-inflammatory effects of calcitriol: implications for prostate cancer chemoprevention and treatment. Endocr. Relat. Cancer. 2010;17 doi: 10.1677/ERC-09-0139. [DOI] [PubMed] [Google Scholar]
- Kuroki T., Sasaki K., Chida K., Abe E., Suda T. vol. 74. 1983. 1 alpha,25-Dihydroxyvitamin D3 Markedly Enhances Chemically-induced Transformation in BALB 3T3 Cells. Gan; pp. 611–614. [PubMed] [Google Scholar]
- Le Tourneau C., Razak A.R., Gan H.K., Pop S., Diéras V., Tresca P., Paoletti X. Heterogeneity in the definition of dose-limiting toxicity in phase I cancer clinical trials of molecularly targeted agents: a review of the literature. Eur. J. Cancer. 2011;40:1468–1475. doi: 10.1016/j.ejca.2011.03.016. [DOI] [PubMed] [Google Scholar]
- Lechner D., Manhardt T., Bajna E., Posner G.H., Cross H.S. A 24-phenylsulfone analog of vitamin D inhibits 1alpha,25-dihydroxyvitamin D(3) degradation in vitamin D metabolism-competent cells. J. Pharmacol. Exp. Ther. 2007;320:1119–1126. doi: 10.1124/jpet.106.115451. [DOI] [PubMed] [Google Scholar]
- Lee L.R., Teng P.N., Nguyen H., Hood B.L., Kavandi L., Wang G. Progesterone enhances calcitriol antitumor activity by upregulating vitamin D receptor expression and promoting apoptosis in endometrial cancer cells. Cancer Prev. Res. (Phila.) 2013;6:731–743. doi: 10.1158/1940-6207.CAPR-12-0493. [DOI] [PubMed] [Google Scholar]
- Limaye S., Posner M., Krane J., Fonfria M., Lorch J., Dillon D., Shreenivas A., Tishler R., Haddad R. Trastuzumab for the treatment of salivary duct carcinoma. Oncologist. 2013;18:294–300. doi: 10.1634/theoncologist.2012-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G., Wilding G., Staab M.J., Horvath D., Miller K., Dresen A. Phase II study of 1alpha-hydroxyvitamin D(2) in the treatment of advanced androgen-independent prostate cancer. Clin. Cancer Res. 2003;9:4077–4083. [PubMed] [Google Scholar]
- Liu J., Dong Y., Lu C. Meta-analysis of the correlation between vitamin D and lung cancer risk and outcomes. Oncotarget. 2017;8:81040–81051. doi: 10.18632/oncotarget.18766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou Y.R., Tuohimaa P. Androgen enhances the antiproliferative activity of vitamin D3 by suppressing 24-hydroxylase expression in LNCaP cells. J. Steroid Biochem. Mol. Biol. 2006;99:44–49. doi: 10.1016/j.jsbmb.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Ly L.H., Zhao X.Y., Holloway L., Feldman D. Liarozole acts synergistically with 1alpha,25-dihydroxyvitamin D3 to inhibit growth of DU 145 human prostate cancer cells by blocking 24-hydroxylase activity. Endocrinology. 1999;140:2071–2076. doi: 10.1210/endo.140.5.6698. [DOI] [PubMed] [Google Scholar]
- Ma Y., Zhang P., Wang F., Yang J., Liu Z., Qin H. Association between vitamin D and risk of colorectal cancer: a systematic review of prospective studies. J. Clin. Oncol. 2011;29:3775–3782. doi: 10.1200/JCO.2011.35.7566. [DOI] [PubMed] [Google Scholar]
- Ma Y., Yu W.D., Hidalgo A.A., Luo W., Delansorne R., Johnson C.S. Inecalcitol, an analog of 1,25D3, displays enhanced antitumor activity through the induction of apoptosis in a squamous cell carcinoma model system. Cell Cycle. 2013;12:743–752. doi: 10.4161/cc.23846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire O., Pollock C., Martin P., Owen A., Smyth T., Doherty D. Regulation of CYP3A4 and CYP3A5 expression and modulation of “intracrine” metabolism of androgens in prostate cells by liganded vitamin D receptor. Mol. Cell. Endocrinol. 2012;364:54–64. doi: 10.1016/j.mce.2012.08.007. [DOI] [PubMed] [Google Scholar]
- Manolagas S.C., Haussler M.R., Deftos L.J. 1,25-dihydroxyvitamin D3 receptors in cancer. Lancet. 1980;1:828. doi: 10.1016/s0140-6736(80)91332-x. [DOI] [PubMed] [Google Scholar]
- McCarthy D.M., San Miguel J.F., Freake H.C., Green P.M., Zola H., Catovsky D., Goldman J.M. 1,25-dihydroxyvitamin D3 inhibits proliferation of human promyelocytic leukaemia (HL60) cells and induces monocyte-macrophage differentiation in HL60 and normal human bone marrow cells. Leuk. Res. 1983;7:51–55. doi: 10.1016/0145-2126(83)90057-7. [DOI] [PubMed] [Google Scholar]
- McGuire T.F., Trump D.L., Johnson C.S. Vitamin D(3)-induced apoptosis of murine squamous cell carcinoma cells. Selective induction of caspase-dependent MEK cleavage and up-regulation of MEKK-1. J. Biol. Chem. 2001;276:26365–26373. doi: 10.1074/jbc.M010101200. [DOI] [PubMed] [Google Scholar]
- Medioni J., Deplanque G., Ferrero J., Maurina T., Rodier J.P., Raymond E. Dose-finding and efficacy phase II study of inecalcitol, a new VDR agonist, in combination with docetaxel-prednisone regimen for castration-resistant prostate cancer (CRPC) patients (pts) J. Clin. Oncol. 2011;29:142–146. [Google Scholar]
- Medioni J., Deplanque G., Ferrero J.M., Maurina T., Rodier J.M., Raymond E. Phase I safety and pharmacodynamic of inecalcitol, a novel VDR agonist with docetaxel in metastatic castration-resistant prostate cancer patients. Clin. Cancer Res. 2014;20:4471–4477. doi: 10.1158/1078-0432.CCR-13-3247. [DOI] [PubMed] [Google Scholar]
- Mernitz H., Smith D.E., Wood R.J., Russell R.M., Wang X.D. Inhibition of lung carcinogenesis by 1alpha,25-dihydroxyvitamin D3 and 9-cis retinoic acid in the A/J mouse model: evidence of retinoid mitigation of vitamin D toxicity. Int. J. Cancer. 2007;120:1402–1419. doi: 10.1002/ijc.22462. [DOI] [PubMed] [Google Scholar]
- Miyata Y., Ohba K., Matsuo T., Watanabe S., Hayashi T., Sakai H. Tumor-associated stromal cells expressing E-prostanoid 2 or 3 receptors in prostate cancer: correlation with tumor aggressiveness and outcome by angiogenesis and lymphangiogenesis. Urology. 2013;81:136–142. doi: 10.1016/j.urology.2012.08.014. [DOI] [PubMed] [Google Scholar]
- Miyaura C., Abe E., Kuribayashi T., Tanaka H., Konno K., Nishii Y., Suda T. 1 alpha,25-Dihydroxyvitamin D3 induces differentiation of human myeloid leukemia cells. Biochem. Biophys. Res. Commun. 1981;102:937–943. doi: 10.1016/0006-291x(81)91628-4. [DOI] [PubMed] [Google Scholar]
- Mohapatra S., Saxena A., Gandhi G., Koner B.C., Singh T., Ray P.C. Does vitamin D mediate inhibition of epithelial ovarian cancer by modulating cytokines? Clin. Transl. Oncol. 2015;17:590–595. doi: 10.1007/s12094-015-1281-3. [DOI] [PubMed] [Google Scholar]
- Moqattash S., Lutton J.D., Chiao J.W., Levere R.D. Abolition of L1210 clonogeneticy and G1 arrest by retinoic acid and 1,25-dihydroxyvitamin D3. Cancer Lett. 1985;27:125–134. doi: 10.1016/0304-3835(85)90101-6. [DOI] [PubMed] [Google Scholar]
- Moreno J., Krishnan A.V., Swami S., Nonn L., Peehl D.M., Feldman D. Regulation of prostaglandin metabolism by calcitriol attenuates growth stimulation in prostate cancer cells. Cancer Res. 2005;65:7917–7925. doi: 10.1158/0008-5472.CAN-05-1435. [DOI] [PubMed] [Google Scholar]
- Morris M.J., Smaletz O., Solit D., Kelly W.K., Slovin S., Flombaum C. High-dose calcitriol, zoledronate, and dexamethasone for the treatment of progressive prostate carcinoma. Cancer. 2004;100:1868–1875. doi: 10.1002/cncr.20185. [DOI] [PubMed] [Google Scholar]
- Mouratidis P.X., Dalgleish A.G., Colston K.W. Investigation of the mechanisms by which EB1089 abrogates apoptosis induced by 9-cis retinoic acid in pancreatic cancer cells. Pancreas. 2006;32:93–100. doi: 10.1097/01.mpa.0000191648.47667.4f. [DOI] [PubMed] [Google Scholar]
- Muindi J.R., Peng Y., Potter D.M., Hershberger P.A., Tauch J.S., Capozzoli M.J. Pharmacokinetics of high-dose oral calcitriol: results from a phase 1 trial of calcitriol and paclitaxel. Clin. Pharmacol. Ther. 2002;72:648–659. doi: 10.1067/mcp.2002.129305. [DOI] [PubMed] [Google Scholar]
- Muindi J.R., Modzelewski R.A., Peng Y., Trump D.L., Johnson C.S. Pharmacokinetics of 1alpha,25-dihydroxyvitamin D3 in normal mice after systemic exposure to effective and safe antitumor doses. Oncology. 2004;66:62–66. doi: 10.1159/000076336. [DOI] [PubMed] [Google Scholar]
- Muindi J.R., Potter D.M., Peng Y., Johnson C.S., Trump D.L. Pharmacokinetics of liquid calcitriol formulation in advanced solid tumor patients: comparison with caplet formulation. Cancer Chemother. Pharmacol. 2005;56:492–496. doi: 10.1007/s00280-005-1015-2. [DOI] [PubMed] [Google Scholar]
- Muindi J.R., Johnson C.S., Trump D.L., Christy R., Engler K.L., Fakih M.G. A phase I and pharmacokinetics study of intravenous calcitriol in combination with oral dexamethasone and gefitinib in patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2009;65:33–40. doi: 10.1007/s00280-009-1000-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muindi J.R., Yu W.D., Ma Y., Engler K.L., Kong R.X., Trump D.L. CYP24A1 inhibition enhances the antitumor activity of calcitriol. Endocrinology. 2010;151:4301–4312. doi: 10.1210/en.2009-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murayama E., Miyamoto K., Kubodera N., Mori T., Matsunaga I. Synthetic studies of vitamin D3 analogues. VIII. Synthesis of 22-oxavitamin D3 analogues. Chem. Pharm. Bull.(Tokyo) 1986;34:4410–4413. doi: 10.1248/cpb.34.4410. [DOI] [PubMed] [Google Scholar]
- Murphy L.C., Wild J., Posen S., Stone G. 25-Hydroxycholecalciferol receptors in human breast cancer. Br. J. Cancer. 1979;39:531–535. doi: 10.1038/bjc.1979.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narvaez C.J., Welsh J. Differential effects of 1,25-dihydroxyvitamin D3 and tetradecanoylphorbol acetate on cell cycle and apoptosis of MCF-7 cells and a vitamin D3-resistant variant. Endocrinology. 1997;138:4690–4698. doi: 10.1210/endo.138.11.5545. [DOI] [PubMed] [Google Scholar]
- Neale R.E., Armstrong B.K., Baxter C., Duarte Romero B., Ebeling P., English D.R., Kimlin M.G., McLeod D.S., O Connell R.L., van der Pols J.C., Venn A.J., Webb P.M., Whiteman D.C., Wockner L. The D-Health Trial: a randomized trial of vitamin D for prevention of mortality and cancer. Contemp. Clin. Trials. 2016;48:83–90. doi: 10.1016/j.cct.2016.04.005. [DOI] [PubMed] [Google Scholar]
- Okamoto R., Delansorne R., Wakimoto N., Doan N.B., Akagi T., Shen M. Inecalcitol, an analog of 1alpha,25(OH)(2) D(3), induces growth arrest of androgen-dependent prostate cancer cells. Int. J. Cancer. 2012;130:2464–2473. doi: 10.1002/ijc.26279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okereke O.I., Reynolds C.F., 3rd, Mischoulon D., Chang G., Cook N.R., Copeland T., Friedenberg G., Buring J.E., Manson J.E. The VITamin D and OmegA-3 TriaL-Depression Endpoint Prevention (VITAL-DEP): rationale and design of a large-scale ancillary study evaluating vitamin D and marine omega-3 fatty acid supplements for prevention of late-life depression. Contemp. Clin. Trials. 2018;17 doi: 10.1016/j.cct.2018.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson I., Gullberg U., Ivhed I., Nilsson K. Induction of differentiation of the human histiocytic lymphoma cell line U-937 by 1 alpha,25-dihydroxycholecalciferol. Cancer Res. 1983;43:5862–5867. [PubMed] [Google Scholar]
- Ordóñez-Mena J.M., Schöttker B., Haug U. Serum 25-hydroxyvitamin d and cancer risk in older adults: results from a large German prospective cohort study. Cancer Epidemiol. Biomark. Prev. 2013;22:905–916. doi: 10.1158/1055-9965.EPI-12-1332. [DOI] [PubMed] [Google Scholar]
- Ordóñez-Mena J.M., Schöttker B., Fedirko V. Pre-diagnostic vitamin D concentrations and cancer risks in older individuals: an analysis of cohorts participating in the CHANCES consortium. Eur. J. Epidemiol. 2016;31:311–323. doi: 10.1007/s10654-015-0040-7. [DOI] [PubMed] [Google Scholar]
- Osborn J.L., Schwartz G.G., Smith D.C., Bahnson R., Day R., Trump D.L. Phase II trial of oral 1,25-dihydroxyvitamin D (calcitriol) in hormone refractory prostate cancer. Urol. Oncol. 1995;1:195–198. doi: 10.1016/1078-1439(95)00061-5. [DOI] [PubMed] [Google Scholar]
- Ostrem V.K., Tanaka Y., Prahl J., DeLuca H.F., Ikekawa N. 24- and 26-homo-1,25-dihydroxyvitamin D3: preferential activity in inducing differentiation of human leukemia cells HL-60 in vitro. Proc. Natl. Acad. Sci. U. S. A. 1987;84:2610–2614. doi: 10.1073/pnas.84.9.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peehl D.M., Feldman D. Interaction of nuclear receptor ligands with the vitamin D signaling pathway in prostate cancer. J. Steroid Biochem. Mol. Biol. 2004;92:307–315. doi: 10.1016/j.jsbmb.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Peehl D.M., Seto E., Hsu J.Y., Feldman D. Preclinical activity of ketoconazole in combination with calcitriol or the vitamin D analogue EB 1089 in prostate cancer cells. J. Urol. 2002;168:1583–1588. doi: 10.1097/01.ju.0000030158.18335.84. [DOI] [PubMed] [Google Scholar]
- Petrylak D.P., Tangen C.M., Hussain M.H., Lara P.N., Jr., Jones J.A., Taplin M.E., Burch P.A., Berry D., Moinpour C., Kohli M., Benson M.C., Small E.J., Raghavan D., Crawford E.D. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N. Engl. J. Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- Pintado C.O., Carracedo J., Rodriguez M., Perez-Calderon R., Ramirez R. 1 alpha, 25-dihydroxyvitamin D3 (calcitriol) induces apoptosis in stimulated T cells through an IL-2 dependent mechanism. Cytokine. 1996;8:342–345. doi: 10.1006/cyto.1996.0047. [DOI] [PubMed] [Google Scholar]
- Potter G.K., Mohamed A.N., Dracopoli N.C., Groshen S.L., Shen R.N., Moore M.A. Action of 1,25-(OH)2D3 in nude mice bearing transplantable human myelogenous leukemic cell lines. Exp. Hematol. 1985;13:722–732. [PubMed] [Google Scholar]
- Ramnath N., Daignault-Newton S., Dy G.K., Muindi J.R., Adjei A., Elingrod V.L. A phase I/II pharmacokinetic and pharmacogenomic study of calcitriol in combination with cisplatin and docetaxel in advanced non-small-cell lung cancer. Cancer Chemother. Pharmacol. 2013;71:1173–1182. doi: 10.1007/s00280-013-2109-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A., Woodruff R.D., Wade W.N., Kute T.E., Cramer S.D. Genistein and vitamin D synergistically inhibit human prostatic epithelial cell growth. J. Nutr. 2002;132:3191–3194. doi: 10.1093/jn/131.10.3191. [DOI] [PubMed] [Google Scholar]
- Rodriguez G.C., Turbov J., Rosales R., Yoo J., Hunn J., Zappia K.J. Progestins inhibit calcitriol-induced CYP24A1 and synergistically inhibit ovarian cancer cell viability: an opportunity for chemoprevention. Gynecol. Oncol. 2016;143:159–167. doi: 10.1016/j.ygyno.2016.04.022. [DOI] [PubMed] [Google Scholar]
- Rubin D., Levij I.S. Suppression by vitamins D2 and D3 of hamster cheek pouch carcinoma induced with 9,10-dimethyl-1,2-benzanthracene with a discussion of the role of intracellular calcium in the development of tumors. Pathol. Microbiol. 1973;39:446–460. [PubMed] [Google Scholar]
- Sano Y., Kogashiwa Y., Araki R., Enoki Y., Ikeda T., Yoda T., Nakahira M., Sugasawa M. Correlation of inflammatory markers, survival, and COX2 expression in oral cancer and implications for prognosis. Otolaryngol. Head Neck Surg. 2018;1 doi: 10.1177/0194599817745284. [DOI] [PubMed] [Google Scholar]
- Sato T., Takusagawa K., Asoo N., Konno K. Antitumor effect of 1 alpha-hydroxyvitamin D3. Tohoku J. Exp. Med. 1982;138:445–446. doi: 10.1620/tjem.138.445. [DOI] [PubMed] [Google Scholar]
- Sato T., Takusagawa K., Asoo N., Konno K. Effect of 1 alpha-hydroxyvitamin D3 on metastasis of rat ascites hepatoma K-231. Br. J. Cancer. 1984;50:123–125. doi: 10.1038/bjc.1984.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher H.I., Jia X., Chi K., de Wit R., Berry W.R., Albers P. Randomized, open-label phase III trial of docetaxel plus high-dose calcitriol versus docetaxel plus prednisone for patients with castration-resistant prostate cancer. J. Clin. Oncol. 2011;29:2191–2198. doi: 10.1200/JCO.2010.32.8815. [DOI] [PubMed] [Google Scholar]
- Schuster I., Egger H., Nussbaumer P., Kroemer R.T. Inhibitors of vitamin D hydroxylases: structure-activity relationships. J. Cell. Biochem. 2003;88:372–380. doi: 10.1002/jcb.10365. [DOI] [PubMed] [Google Scholar]
- Schwartz G.G., Hall M.C., Stindt D., Patton S., Lovato J., Torti F.M. Phase I/II study of 19-nor-1alpha-25-dihydroxyvitamin D2 (paricalcitol) in advanced, androgen-insensitive prostate cancer. Clin. Cancer Res. 2005;11:8680–8685. doi: 10.1158/1078-0432.CCR-05-1237. [DOI] [PubMed] [Google Scholar]
- Seo Y.K., Mirkheshti N., Song C.S., Kim S., Dodds S., Ahn S.C. SULT2B1b sulfotransferase: induction by vitamin D receptor and reduced expression in prostate cancer. Mol. Endocrinol. 2013;27:925–939. doi: 10.1210/me.2012-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifi N. Minireview: androgen metabolism in castration-resistant prostate cancer. Mol. Endocrinol. 2013;27(5):708–714. doi: 10.1210/me.2013-1007. (Epub 2013 Apr 16) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher E., Eisman J.A., Moseley J.M., Martin T.J. Whole-cell uptake and nuclear localization of 1,25-dihydroxycholecalciferol by breast cancer cells (T47 D) in culture. Biochem. J. 1981;200:315–320. doi: 10.1042/bj2000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simboli-Campbell M., Narvaez C.J., van Weelden K., Tenniswood M., Welsh J. Comparative effects of 1,25(OH)2D3 and EB1089 on cell cycle kinetics and apoptosis in MCF-7 breast cancer cells. Breast Cancer Res. Treat. 1997;42:31–41. doi: 10.1023/a:1005772432465. [DOI] [PubMed] [Google Scholar]
- Skaaby T., Husemoen L.L., Thuesen B.H. Prospective population-based study of the association between serum 25-hydroxyvitamin-d levels and the incidence of specific types of cancer. Cancer Epidemiol. Biomark. Prev. 2014;23:1220–1229. doi: 10.1158/1055-9965.EPI-14-0007. [DOI] [PubMed] [Google Scholar]
- Skowronski R.J., Peehl D.M., Feldman D. Actions of vitamin D3, analogs on human prostate cancer cell lines: comparison with 1,25-dihydroxyvitamin D3. Endocrinology. 1995;136:20–26. doi: 10.1210/endo.136.1.7530193. [DOI] [PubMed] [Google Scholar]
- Smith D.C., Johnson C.S., Freeman C.C., Muindi J., Wilson J.W., Trump D.L.A. Phase I trial of calcitriol (1,25-dihydroxycholecalciferol) in patients with advanced malignancy. Clin. Cancer Res. 1999;5:1339–1345. [PubMed] [Google Scholar]
- Solomon B., Mok T., Kim D., Wu Y., Nakagawa K., Mekhail T., Felip E., Cappuzzo F., Paolini J., Usari T., Iyer S., Reisman A., Wilner K., Tursi J., Blackhall F., PROFILE 1014 Investigators First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N. Engl. J. Med. 2014;371:2167–2177. doi: 10.1056/NEJMoa1408440. [DOI] [PubMed] [Google Scholar]
- Srinivas S., Feldman D. A phase II trial of calcitriol and naproxen in recurrent prostate cancer. Anticancer Res. 2009;29:3605–3610. [PubMed] [Google Scholar]
- Sun L., Chen K., Jiang Z., Chen X., Ma J., Ma Q., Duan W. Indometacin inhibits the proliferation and activation of human pancreatic stellate cells through the downregulation of COX-2. Oncol. Rep. 2018;39:2243–2251. doi: 10.3892/or.2018.6321. [DOI] [PubMed] [Google Scholar]
- Swami S., Krishnan A.V., Peehl D.M., Feldman D. Genistein potentiates the growth inhibitory effects of 1,25-dihydroxyvitamin D3 in DU145 human prostate cancer cells: role of the direct inhibition of CYP24 enzyme activity. Mol. Cell. Endocrinol. 2005;241:49–61. doi: 10.1016/j.mce.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Tanaka H., Abe E., Miyaura C., Kuribayashi T., Konno K., Nishii Y., Suda T. 1 alpha,25-Dihydroxycholecalciferol and a human myeloid leukaemia cell line (HL-60) Biochem. J. 1982;204:713–719. doi: 10.1042/bj2040713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock I.F., de Wit R., Berry W.R., Horti J., Pluzanska A., Chi K.N., Oudard S., Théodore C., James N.D., Turesson I., Rosenthal M.A., Eisenberger M.A., 327 Investigators T.A.X. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N. Engl. J. Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- Tran E., Robbins P.F., Rosenberg S.A. ‘Final common pathway’ of human cancer immunotherapy: targeting random somatic mutations. Nat. Immunol. 2017;18:255–262. doi: 10.1038/ni.3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trump D.L., Hershberger P.A., Bernardi R.J., Ahmed S., Muindi J., Fakih M. Anti-tumor activity of calcitriol: pre-clinical and clinical studies. J. Steroid Biochem. Mol. Biol. 2004;89-90:519–526. doi: 10.1016/j.jsbmb.2004.03.068. [DOI] [PubMed] [Google Scholar]
- Trump D.L., Potter D.M., Muindi J., Brufsky A., Johnson C.S. Phase II trial of high-dose, intermittent calcitriol (1,25 dihydroxyvitamin D3) and dexamethasone in androgen-independent prostate cancer. Cancer. 2006;106:2136–2142. doi: 10.1002/cncr.21890. [DOI] [PubMed] [Google Scholar]
- Van Belle T.L., Vanherwegen A.S., Feyaerts D., De Clercq P., Verstuyf A., Korf H., Gysemans C., Mathieu C. 1,25-Dihydroxyvitamin D3 and its analog TX527 promote a stable regulatory T cell phenotype in T cells from type 1 diabetes patients. PLoS One. 2014;3(9) doi: 10.1371/journal.pone.0109194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Belle T.L., Vanherwegen A.S., Feyaerts D., De Clercq P., Verstuyf A., Korf H. 1,25-Dihydroxyvitamin D3 and its analog TX527 promote a stable regulatory T cell phenotype in T cells from type 1 diabetes patients. PLoS One. 2014;9 doi: 10.1371/journal.pone.0109194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verlinden L., Verstuyf A., Convents R., Marcelis S., Van Camp M., Bouillon R. Action of 1,25(OH)2D3 on the cell cycle genes, cyclin D1, p21 and p27 in MCF-7 cells. Mol. Cell. Endocrinol. 1998;142:57–65. doi: 10.1016/s0303-7207(98)00117-8. [DOI] [PubMed] [Google Scholar]
- Verlinden L., Verstuyf A., Van Camp M., Marcelis S., Sabbe K., Zhao X.Y. Two novel 14-Epi-analogues of 1,25-dihydroxyvitamin D3 inhibit the growth of human breast cancer cells in vitro and in vivo. Cancer Res. 2000;60:2673–2679. [PubMed] [Google Scholar]
- Verlinden L., Leyssens C., Beullens I., Marcelis S., Mathieu C., De Clercq P. The vitamin D analog TX527 ameliorates disease symptoms in a chemically induced model of inflammatory bowel disease. J. Steroid Biochem. Mol. Biol. 2013;136:107–111. doi: 10.1016/j.jsbmb.2012.09.017. [DOI] [PubMed] [Google Scholar]
- Wagner D., Trudel D., Van der Kwast T., Nonn L., Giangreco A.A., Li D. Randomized clinical trial of vitamin D3 doses on prostatic vitamin D metabolite levels and ki67 labeling in prostate cancer patients. J. Clin. Endocrinol. Metab. 2013;98:1498–1507. doi: 10.1210/jc.2012-4019. [DOI] [PubMed] [Google Scholar]
- Wang Q.M., Jones J.B., Studzinski G.P. Cyclin-dependent kinase inhibitor p27 as a mediator of the G1-S phase block induced by 1,25-dihydroxyvitamin D3 in HL60 cells. Cancer Res. 1996;56:264–267. [PubMed] [Google Scholar]
- Wang W., Bergh A., Damber J.E. Cyclooxygenase-2 expression correlates with local chronic inflammation and tumor neovascularization in human prostate cancer. Clin. Cancer Res. 2005;11:3250–3256. doi: 10.1158/1078-0432.CCR-04-2405. [DOI] [PubMed] [Google Scholar]
- Welsh J. Induction of apoptosis in breast cancer cells in response to vitamin D and antiestrogens. Biochem. Cell Biol. 1994;72:537–545. doi: 10.1139/o94-072. [DOI] [PubMed] [Google Scholar]
- Yee S.W., Simons C. Synthesis and CYP24 inhibitory activity of 2-substituted-3,4-dihydro-2H-naphthalen-1-one (tetralone) derivatives. Bioorg. Med. Chem. Lett. 2004;14:5651–5654. doi: 10.1016/j.bmcl.2004.08.040. [DOI] [PubMed] [Google Scholar]
- Yee S.W., Campbell M.J., Simons C. Inhibition of vitamin D3 metabolism enhances VDR signalling in androgen-independent prostate cancer cells. J. Steroid Biochem. Mol. Biol. 2006;98:228–235. doi: 10.1016/j.jsbmb.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Yin L., Ordóñez-Mena J.M., Chen T., Schöttker B., Arndt V., Brenner H. Circulating 25-hydroxyvitamin D serum concentration and total cancer incidence and mortality: a systematic review and meta-analysis. Prev. Med. 2013;57:753–764. doi: 10.1016/j.ypmed.2013.08.026. [DOI] [PubMed] [Google Scholar]
- Yoshimura R., Sano H., Masuda C., Kawamura M., Tsubouchi Y., Chargui J. Expression of cyclooxygenase-2 in prostate carcinoma. Cancer. 2000;89:589–596. [PubMed] [Google Scholar]
- Zha S., Gage W.R., Sauvageot J., Saria E.A., Putzi M.J., Ewing C.M. Cyclooxygenase-2 is up-regulated in proliferative inflammatory atrophy of the prostate, but not in prostate carcinoma. Cancer Res. 2001;61:8617–8623. [PubMed] [Google Scholar]
- Zhang Q., Kanterewicz B., Buch S., Petkovich M., Parise R., Beumer J. CYP24 inhibition preserves 1alpha,25-dihydroxyvitamin D(3) anti-proliferative signaling in lung cancer cells. Mol. Cell. Endocrinol. 2012;355:153–161. doi: 10.1016/j.mce.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Tan B.K., Marcelis S., Verstuyf A., Bouillon R. Enhancement of antiproliferative activity of 1alpha,25-dihydroxyvitamin D3 (analogs) by cytochrome P450 enzyme inhibitors is compound- and cell-type specific. J. Steroid Biochem. Mol. Biol. 1996;57:197–202. doi: 10.1016/0960-0760(95)00256-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency document