Fig. 5.
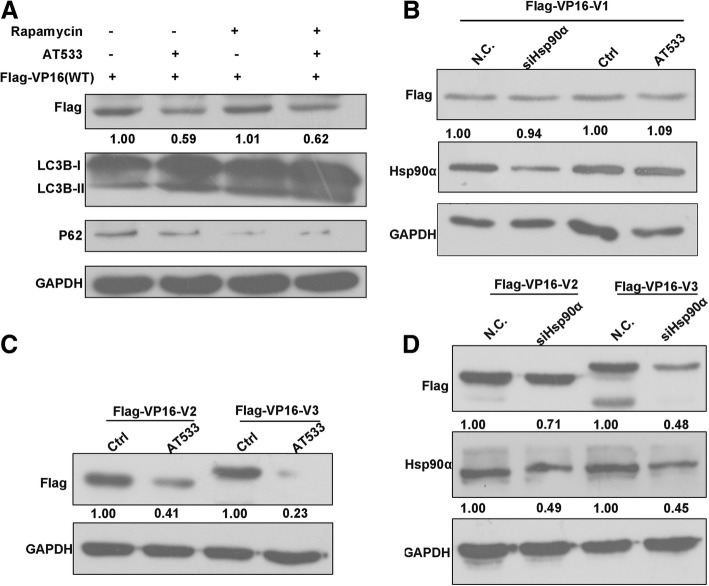
Hsp90 inhibition-induced VP16 degradation depends on the conserved core domain of VP16. a Treatment with rapamycin failed to lead to VP16 degradation. Vero cells were transfected with FLAG-VP16 plasmid (3 μg) for 36 h, treated with rapamycin (250 nM) for 10 h, and then treated with AT533 (2 μM) for another 2 h. Total proteins were extracted and analyzed by western blotting. b Hsp90α knockdown and inhibition did not affect the level of mutant V1. Vero cells were cotransfected with siHsp90α-2 (100 nM) and the mutant FLAG-VP16 plasmid V1 (3 μg) for 48 h, and total proteins were extracted for western blot analysis (left). Vero cells were transfected with the mutant FLAG-VP16 plasmid V1 (3 μg) for 48 h and then treated with AT533 (2 μM) for 2 h. Total proteins were extracted and analyzed by western blotting (right). c Knockdown of Hsp90α reduced the levels of truncated mutants V2 and V3. SiHsp90α-2 (100 nM) was cotransfected with the mutant plasmid V2 or V3 (3 μg) into Vero cells for 48 h, and the levels of the indicated proteins were analyzed by western blotting. d Vero cells were transfected with the truncated mutant plasmid V2 or V3 (3 μg) for 46 h and then treated with AT533 (2 μM) for another 2 h. Total protein was extracted and the expression levels of the proteins of interest were analyzed by western blotting