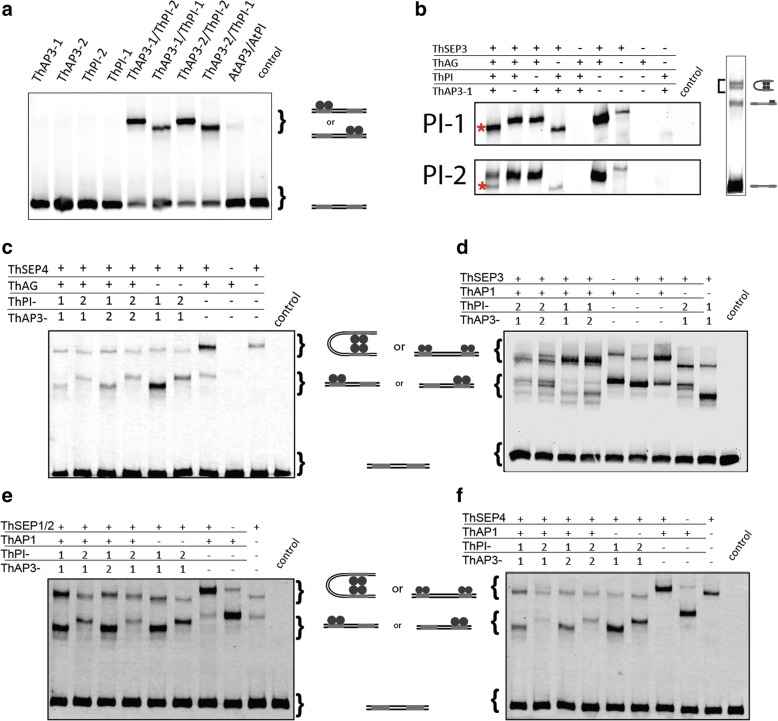
Fig. 6.
DNA-binding protein-complexes formed by T. hassleriana B-class proteins. In (a), homo- and heterodimerization of ThAP3 and ThPI (b) Complexes formed with ThAG, ThSEP3 (Th01528), ThAP3–1 and either of the two ThPI paralogs. The figure shows only the higher order complexes (tetramers); in the right image this part compared to the whole gel is indicated. In (c), EMSA for protein complexes with ThAG and a ThSEP4 paralog (Th21984). (d, e, f) EMSAs testing the formation of a DNA-binding protein complex of the B-class dimers with a ThAP1 paralog (Th13754) and with different ThSEP paralogs: ThSEP3 (Th1528) (d), ThSEP1/2 (Th2854) (e), and ThSEP4 (Th21984) (f). For all experiments, a promoter fragment from the A. thaliana SEP3 promoter was used as probe (Smaczniak et al., 2012a). The control is an empty-vector control, in which no MADS protein production is expected