Abstract
Each year, large numbers of bats move across Europe between their summer and winter areas, yet even though many of them are endangered and legally protected, we are unaware about many aspects of their migratory behaviour. Here, taking Nyctalus noctula as a model species, we used stable hydrogen isotopic values in fur (δ2Hf) as an endogenous marker to shed light on the migratory behaviour of more than 1000 bats from hibernacula across Central Europe. Specifically, we asked the following questions: how flexible is migration in temperate zone bats? Which general migration pattern do noctule bats follow? How repeatable and thus predictable is the migratory behaviour of individuals? Do morphological correlates of migration occur in bats? Our study confirmed that noctule bats engage in partial and female-biased migration across Europe, suggesting the strongest migration pressures for northern populations. Further, we revealed a combination of partial and differential migration patterns with highly variable migration distances which lead to a pronounced mixing of different source populations in hibernacula where mating occurs. Most individuals were consistent in their migration strategy over time, i.e. 86% could be repeatedly assigned to either long-distance or regional origin across years. This is consistent with our finding that the between-individual component explained 84% of the variation in δ2Hf values, suggesting specialized individual migratory behaviours and a strong natal philopatry. We discovered a positive correlation between forearm length and migration distance and support for sex-specific effects of migration on body condition. Our study elucidated migration patterns over large geographical scales, demonstrating that considerable numbers of migratory bats originating from distant populations depend on hibernacula across Central Europe, calling for international conservation management.
Keywords: isoscape origin model, stable isotope analysis, IsoriX, deuterium, fur
1. Introduction
The need to survive and reproduce is considered a main driver of seasonal migrations, seeking optimal conditions during breeding while ensuring survival during the rest of the year. As a consequence, migration is most pronounced in regions with strong seasonality such as the temperate zone. A complete migration depicts the simplest case of migration with all individuals of a species behaving in a similar way, e.g. all animals migrate north in spring and return south in winter for animals of the Northern Hemisphere [1]. However, most realized migration patterns are more complex, displaying various degrees of inter- and intra-population variation, which is thought to occur for different reasons. For example, breeding populations from northern latitudes may depend on wintering areas with milder temperatures or higher winter food availability, while populations at lower latitudes may thrive in their habitat year round. Or animals may differ in some physiological parameters such as energy expenditure of reproduction, which may force only parts of a population (e.g. one sex or certain age classes) to migrate. In birds, typical migration patterns with inter-population variation include, for example, leap-frog migration, where long-distance migratory populations overfly areas of potentially suitable habitats occupied by resident populations ([2], figure 1). Additionally, some migratory birds show breeding displacement, where one population displaces the other ([2], figure 1). However, the migratory tendency may not only vary between populations but also between individuals of the same population; a pattern called partial migration (e.g. [3–5]). Furthermore, within populations, animals may not only exhibit differences in migratory tendency per se but also in migration distances, a phenomenon called differential migration [6,7]. Lastly, individuals with different morphologies may vary in their migratory behaviour, e.g. long-winged birds are known to cover longer distances than short-winged conspecifics [8,9].
Figure 1.
Schematic representation of alternative patterns of noctule bat migration in Europe. Blue-shaded circles depict populations (full circle = 100% of animals) within areas (A–D) of distinct environmental isotopic composition (so-called isoclines). (a) Leap-frog migration: animals from northern breeding populations overfly more central populations to reach southernmost wintering areas. (b) Post-breeding displacement: post-breeding animals of northern populations migrate south and displace animals from these areas which in turn move further south for hibernation. (c) Partial migration variant 1: only a proportion of animals from northern populations migrates south to hibernacula at varying distances from the breeding site. (d) Partial migration variant 2: migratory individuals occur in all breeding colonies across Europe joining hibernacula at varying distances from the breeding site.
For birds, two migratory ecophenotypes have been described—obligate and facultative migrants—which are positioned at opposite ends of a continuum [10]. Obligate migration is characterized by regularity, consistency, and predictability across years with respect to timing, directions, and distances [10]. On the contrary, facultative migration may occur as a spontaneous response of birds to adverse environmental conditions. Then, individuals may switch between migratory and sedentary strategies across years depending on the prevailing food availability and climate conditions [10]. However, while individual migration strategies have been studied in detail for many bird species, (e.g. [11]), we have only limited knowledge about migratory strategies in bats, a taxon known to perform long-distance migrations of several thousand kilometres as well [12–14].
Migration in insectivorous bats of the temperate zone consists usually of a seasonal two-way movement between summering and hibernation areas to avoid physiologically challenging climatic conditions by switching to areas with more favourable conditions, e.g. places where insects are more abundant as a food source [14] or where ambient temperature is ideal for hibernation [12]. Consequently, selection for migratory behaviour is generally expected to be strongest at higher latitudes where seasonality is most pronounced [14]. The common noctule bat (Nyctalus noctula), a European tree-dwelling insectivorous bat species, is known to show both sedentary and migratory behaviour [15,16]. Some individuals may cover distances of up to 1600 km during migration [17]. Populations are considered to include partial and differential migrants [12,18].
Studying noctule bat colonies in the Netherlands, Van Heerdt and Sluiter observed migration distances ranging between 60 and 900 km, yet at that early period of banding efforts, the authors could not infer a distinct migration pattern for this species [19,20]. Later, Steffens and colleagues reported that Central European populations of N. noctula do not exhibit pronounced migration behaviour similar to other long-distance migrants such as N. leisleri or Pipistrellus nathusii [15]. Further, they highlighted that populations may consist of residents, migrants, and hibernating individuals in the eastern parts of Germany [15]. Migration distances observed for N. noctula banded in Germany ranged between 200 and 800 km. Further, most individuals from populations from eastern Germany, where banding efforts have focused, hibernated in western and southwestern Germany, Switzerland, and adjacent regions [15].
Banding efforts have mostly taken place in eastern parts of Germany. Accordingly, the picture for noctule bat migration is not yet complete and heavily biased towards sites of previous banding efforts [16]. Further, no comprehensive data are available for bats within a given hibernacula because usually only a few banded individuals are recaptured by chance and because tree hibernacula are usually not monitored on a regular scale. Therefore, it is unclear to what extent local hibernating bats consist of resident and migrant individuals and whether this ratio changes with latitude (figure 1). In addition, no data exist regarding how consistent migratory behaviour is within individual bats and if migratory behaviour is related to certain morphological traits of individuals. A more detailed knowledge of bat migration is strongly needed because increased mortality rates at wind turbines have put migratory bats at risk, despite their protection by U.N. conventions (EUROBATS convention based on agreement from London 1991) and both national and international laws (E.U. Habitat Directive 92/43/CEE; Annexes II and IV; [21,22]).
Here, we used N. noctula as a model species to investigate the migratory behaviour of male and female bats found in hibernacula across Central Europe, ranging from northern Poland to Slovenia. The terminology used to describe migratory behaviours of bats is inconsistent in the literature. Three categories—sedentary, regional migrants, and long-distance migrants—are often in use, albeit with varying meanings. Following Fleming & Eby [14] and Hutterer [16], we consider movements less than 50 km as non-migratory behaviour and bat individuals moving within this range throughout the year as sedentary. Yet, we acknowledge that observed migration distances suggest a continuum of migratory behaviour for noctule bats. In past studies, authors have mostly used an arbitrary distinction between regional and long-distance migrants, usually in response to some methodological constraints. For example, based on the spatial resolution of isoscape origin models in Europe, we have used a threshold distance of 400 km between summer and wintering areas to separate long-distance migrating from regional noctule bats, i.e. bats within this range could not be distinguished using fur stable hydrogen isotope ratios (δ2Hf) ([23]; see electronic supplementary material). For consistency, we will continue working with the terms regional migrants (including local bats) and long-distance migrants.
On the population level, we asked which general migration pattern(s) do noctule bats show in Central Europe (figure 1)? And, does migration vary across a broad geographical range? To answer these two questions, we used isoscape origin models based on δ2Hf to illuminate the connectivity between summering and hibernation areas of noctule bats in Europe. Based on information from banding efforts (e.g. [24]) and our previous studies on N. noctula in northern Germany [18,23], we hypothesized that common noctules are partial migrants. We predicted finding long-distance migrants in all hibernacula but with the majority of individuals being of regional origin. Furthermore, we hypothesized northern populations would experience stronger migration pressure and consequently predicted a decrease in the number of long-distance migrants in hibernacula from north to south. In order to uncover migration patterns more clearly, we took prevailing bird migration patterns as a template to test for consistent patterns in the measured δ2Hf values. We expected that, if migratory noctule bats followed a leap-frog pattern, we would find many individuals with relatively low δ2Hf values (northern origin) in the southernmost regions and a majority of regional individuals in hibernacula of mid-latitudes. However, in the case of displacement migration, we expected to find (a) step-like southward movements for hibernation with characteristic δ2Hf values in the different breeding populations, (b) consistent migration distances (as suggested by δ2Hf values discriminating between wintering and breeding site) and, (c) matching numbers of ‘displacing’ and ‘displaced’ individuals.
On the individual level, we asked how repeatable and thus predictable migratory behaviour of individuals is. Further, we asked if the estimated summer origin of animals is related to certain morphological traits, such as forearm length and body condition. For the latter two questions, we focused on a hibernacula close to Berlin for which we had comprehensive multi-year data, including morphological measures of individually marked noctules. Based on the proposed migratory flexibility, we hypothesized that noctules are facultative migrants. Consequently, we predicted that individuals would readily switch their migration strategy between years and that intra-individual variation in stable isotope signals from different years would be high.
We then tested the explanatory power of specific morphological traits in explaining measured δ2Hf values. We hypothesized that—in analogy to birds—morphological correlates of migration occur in bats [8,9]. Thus, we predicted that individuals with long forearms would preferentially conduct long-distance migrations. We furthermore investigated if bats show condition-dependent migration, similar to birds (e.g. [25]). We predicted migrants to be in significantly better body condition compared to regionals if N. noctula followed condition-dependent migration. Lastly, we tested if male and female noctules differed in their migratory behaviour on a larger geographical scale, because previous banding and isoscape origin studies indicated sex-related differences [15,16,18].
2. Material and methods
(a). Collection and analysis of samples
Between 2007 and 2016, we collected 1170 fur samples of 1078 male and female N. noctula encountered either during hibernacula monitoring activities or while rescuing disturbed winter colonies at the beginning of hibernation in Germany, Switzerland, Poland, and Slovenia (electronic supplementary material, figure S1). Seventy-nine individuals from Berlin-Brandenburg were recaptured at least once in different years. Work was conducted under the following permit numbers: 120/2013 and FIBL1/12 for Switzerland, 86.44-12-0299/20155, 10315/12 and 4440-236-NF/001-2008 for Germany, DOPog-4201-04A-1/2002 for Poland, and 35601-35/2010-6 for Slovenia. For each individual capture, we collected a small tuft of fur from the dorsal pelage, recorded the sex and, at selected sites, measured forearm length (mm) and body mass (g) using a caliper and handheld balance, respectively. We pooled isotopic data from individuals of hibernacula in close proximity (100 km) and considered them as coming from one sampling region. For reasons of simplicity, we refer to ‘hibernacula area’, yet acknowledge that animals may come from several sites within each area. Dry fur samples were sent to the Leibniz Institute for Zoo and Wildlife Research (Berlin) for stable isotope analyses. Technical details related to the analysis of the stable isotope ratios of the non-exchangeable portion of the hydrogen in fur keratin (δ2Hf; ‰) are reported in Popa-Lisseanu et al. [26]) and in the electronic supplementary material. Details on sex (males, females), morphological parameters, δ2Hf values, and sampling region of the hibernating noctule bats can be found in electronic supplementary material, table S1.
(b). Variability of migratory behaviour in noctule bats (migration patterns)
To investigate general migration patterns of noctule bats in Central Europe, we illuminated the connectivity between summering and hibernation areas via isoscape origin models based on δ2Hf values. Isoscape origin modelling uses information on large-scale isotopic patterns in meteoric waters across continents to track migratory movements of animals [18,27] because δ2Hf values reflect the variation of stable hydrogen isotope ratios in precipitation water (δ2Hp) assimilated by animals along the food chain [28]. To delineate the origin of hibernating N. noctula in the δ2Hp isoscape of Europe, we established a relationship between δ2Hf values of local, non-migrating bats and δ2Hp values of the corresponding sites, accounting for potential geospatial assignment errors.
All statistical analyses were performed in R v. 3.4.1 [29] using the R package ‘IsoriX’ (v. 0.4-1) [30]. Briefly, we used δ2Hp values of Europe available from the Global Network of Isotopes in Precipitation [31] to fit a geostatistical mixed model, approximating the relationship between topographic features of a location and its mean annual δ2Hp signature. In ‘IsoriX’, the mean isotopic values and their associated residual dispersion variance are both fitted in a spatially explicit manner. Subsequently, we built a δ2Hp isoscape for Europe (electronic supplementary material, figure S1), using the geospatial model to predict the spatial distribution of δ2Hp values in our area of interest. In a next step, we used a linear mixed-effects model (LMM) to fit δ2Hf values as a linear function of δ2Hp values [30]. The fixed-effect regression equation read: δ2Hp = 33.39 + 1.087 * δ2Hf (see electronic supplementary material, ‘Noctule bat calibration model’ for details). This LMM accounts for the uncertainty stemming from both the calibration data and the inferred isoscape.
For the origin assignment, individuals with similar δ2Hf values were pooled into five (arbitrarily chosen) 10 ‰ bins (A–E; electronic supplementary material, table S2) to facilitate geographical representation. Some individuals (5.7% of samples; n = 63) had δ2Hf values not covered by the range of our calibration model and were therefore excluded (electronic supplementary material, table S2, column X). During the assignment procedure, which controlled for uncertainty stemming from both model fits (geostatistical model and calibration model), we tested for each location across the isoscape to determine if it had a similar isotopic signature as the unknown origin of given individuals [30]. Finally, assignments were visualized highlighting areas with high origin probabilities (figure 2).
Figure 2.
Predicted geographical provenance of about 1100 common noctule bats (Nyctalus noctula) from seven hibernacula (pie charts) in Central Europe. Based on measured stable isotope ratios in the non-exchangeable hydrogen of fur keratin (δ2Hf), animals were assigned to five pre-defined δ2Hf bins (electronic supplementary material, table S2), which were then mapped as areas of likely geographical origin (a–e). Geographical areas marked in green indicate areas of likely summer origin while grey areas indicate areas of unlikely breeding origin. Areas falling outside the distribution range of Nyctalus noctula according to the International Union for Conservation of Nature (IUCN) [32] are overlaid with a striped layer. Further, mountain ranges beyond 400 m elevation were excluded as likely areas of origin because common noctules are a lowland species [17]. Pie charts indicate the proportion of animals from each hibernacula assigned to the specific area of likely origin marked in green.
To test for partial and differential migration, we studied the composition of noctule bats in hibernacula. We identified migratory individuals using the parameters of our calibration model (see above). Based on the regression relationship and the associated uncertainty, individuals were classified as either regional or long-distance migrants if individual δ2Hf values were inside or outside of the 95% confidence interval of the expected δ2Hf values for the specific site, respectively.
We explored whether northern populations experience stronger migration pressure by testing for a decrease in the number of long-distance migrants in hibernacula from north to south. To account for different sample sizes in hibernacula, we used a bootstrapping approach when testing for differences in the proportion of long-distance migrants between hibernacula (see electronic supplementary material).
Moreover, we explored sex-related differences in migratory behaviour within the categories ‘regional’ and ‘long-distance’, testing measured δ2Hf values of regional and long-distance migrants using the t-test and Mann–Whitney–Wilcoxon test, respectively. For this, we considered data from the Berlin-Brandenburg hibernacula area only, because we obtained the largest dataset from there.
(c). Repeatability of individual migratory behaviour
For studying individual migration strategies, we used data of individually marked noctules (n = 120) recaptured in different years in a hibernacula in northeast Germany. Based on our regression model, we distinguished between long-distance and regional migrants for each year of capture. For 79 individuals, we obtained fur samples from at least 2 years and were thus able to monitor migratory behaviour and its variation over time. Finally, we conducted a variance component analysis in RInSp (R package [33]), assuming that if individual migratory behaviours are highly specialized, within-individual variability in δ2Hf values is lower than between-individual variability, i.e. the majority of isotopic variance is explained by between-individual variation [34].
(d). Morphological correlates of migration
To test whether morphology explains migratory distance, we assessed the importance of specific phenotypic morphological traits in explaining the measured δ2Hf values. We fitted an LMM exploring the influence of the explanatory variables ‘forearm length’, ‘body condition’ [body mass (g) divided by forearm length (mm)], and their interaction with ‘sex’ on the dependent variable δ2Hf (see electronic supplementary material).
3. Results
(a). Variability of migratory behaviour in noctule bats (migration patterns)
We studied the likely summer origin of 1078 noctule bats (1170 fur samples) from seven hibernacula in Central Europe. Our isoscape origin models based on δ2Hf values documented that these bats originated from a relatively large geographical area, reaching from Central Europe via Fennoscandia and the eastern Baltic countries to Belarus and Russia (figure 2). Long-distance migrants were found in all hibernacula, albeit at varying numbers (table 1). Multiple pair-wise comparisons (Dunn's test) revealed that the proportions of long-distance migrants varied between hibernacula showing a general decrease from higher to lower latitudes: FC Schleswig-Holstein (38%) > FC North-Rhine-Westphalia (29%) > FC Berlin-Brandenburg (19%) > Poland (16%) > Switzerland (13.7%) > FC Saxony (6.6%) > Slovenia (3.5%) (bootstrap results; but see table 1).
Table 1.
Number, sex (males, females, n.a., not available), and stable isotope ratios in the non-exchangeable hydrogen of fur keratin (δ2Hf; ‰; mean ± s.d.) of migrant and regional common noctules (Nyctalus noctula) found in hibernacula across Central Europe. Note that numbers for FC Berlin-Brandenburg include 92 recaptures of 79 individuals.
| long-distance migrants |
regional |
|||||
|---|---|---|---|---|---|---|
| sampling regions | % | total (m/f/n.a.) | δ2Hf | % | total (m/f/n.a.) | δ2Hf |
| FC Schleswig-Holstein (Germany) | 38 | 95 (22/73/–) | −111.6 ± 8.8 | 62 | 154 (60/94/–) | −92.1 ± 6.1 |
| FC North Rhine-Westphalia (Germany) | 30 | 17 (2/15/–) | −108.6 ± 7.0 | 70 | 40 (20/20/–) | −88.1 ± 7.3 |
| FC Berlin-Brandenburg (Germany) | 19 | 133 (35/89/9) | −116.8 ± 9.2 | 81 | 571 (244/280/47) | −91.4 ± 8.3 |
| FC Saxony (Germany) | 7 | 3 (0/3/–) | −122.4 | 94 | 43 (12/31/–) | −96.4 ± 7.2 |
| Poland | 16 | 6 (2/4/–) | −120.8 ± 4.6 | 84 | 32 (16/16/–) | −100.6 ± 6.8 |
| Switzerland | 14 | 7 (4/3/–) | −127.9 ± 3.6 | 86 | 43 (17/26/–) | −97.6 ± 8.9 |
| Slovenia | 4 | 1 (0/1/–) | −115.8 | 96 | 25 (7/18/–) | −94.6 ± 10.0 |
| ∑ | 22% (262 individuals) | 78% (908 individuals) | ||||
For long-distance migrants, we detected unbalanced sex ratios in all hibernacula, with females outnumbering males in six out of seven sites (table 1). For regional bats, sex ratios were more balanced; however, females were also found more often than males in four out of seven hibernacula (table 1). Significant sex-related differences in δ2Hf values were found for regional individuals (t-test: p < 0.001) but not for long-distance migrants (Wilcoxon: p = 0.26). δ2Hf values of regional females were lower than those of male conspecifics (mean δ2Hf females: −93.1 ‰; mean δ2Hf males: −90.2 ‰).
(b). Repeatability of individual migratory behaviour
Periods between first and last recapture of individually marked noctules ranged between 1 and 12 years (median = 5 years; electronic supplementary material, figure S2). In 41 banded bats, we obtained fur samples only once (20% long-distance migrants, 80% regionals). In 79 other individuals, we obtained a minimum of two fur samples (electronic supplementary material, figure S3). Eighty-six per cent of these noctules showed no variability in their migratory behaviours over time (15% long-distance migrants; 85% regionals) (figure 3). The remaining individuals showed variability over time, i.e. they switched between long-distance and regional migratory behaviours (figure 3). Fourty-eight per cent of long-distance migrants showed no variability in migratory behaviour over time. Furthermore, a variance component analysis indicated that between-individual variation explained 84% of the variation in δ2Hf values and within-individual variation explained 16%.
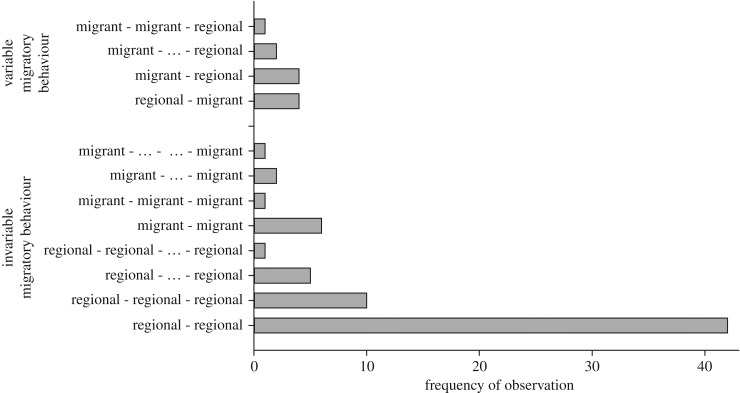
Figure 3.
Variation of migratory behaviour in individual bats recaptured in different years. The summer origin of individuals was identified based on measured stable isotope ratios in the non-exchangeable hydrogen of fur keratin (δ2Hf) for each capture event and individuals were assigned to two categories—long-distance migrant or regional—for the specific year. Assignments were listed for each recapture and years in which bats were absent from the hibernacula are indicated by ‘…’. The majority of common noctule bats (Nyctalus noctula) exhibited invariable migratory behaviour with either only regional (58) or migratory behaviour (10). Only 11 out of 79 individuals showed variable migratory behaviour.
(c). Morphological correlates of migration
The final model in explaining δ2Hf values contained ‘forearm length’, ‘body condition’, ‘sex’, and the interaction between ‘sex’ and ‘body condition’ as significant variables (conditional R2 = 0.70, electronic supplementary material, table S3). Forearm length in both sexes increased with decreasing (more negative) δ2Hf values (electronic supplementary material. figure S4). The effect of δ2Hf values on body condition was sex-specific with male body condition improving with increasing δ2Hf values (typical for regionals) and female body condition showing the opposite trend (electronic supplementary material, figure S4). We did not detect any collinearity in the predictor variables of the LMM (|r| < 0.5). The model containing ‘animal ID’ as the random intercept performed best, as indicated by minimization of Akaike Information Criterion (AIC). Removing the non-significant interaction between ‘sex’ and ‘forearm length’ from the full model had no significant effect according to the likelihood ratio test.
4. Discussion
(a). Variability of migratory behaviour in noctule bats (migration patterns)
Here, we present the first study examining the migratory behaviours of groups of common noctule bats found in hibernacula spread across Central Europe. As predicted, our data demonstrate a high variability of migratory behaviour and thus provide evidence for partial migration in N. noctula not only in northern populations but across Central Europe. Thus, our study substantially expands knowledge beyond the geographical range covered by previous banding efforts [15]. As expected, we found that the majority of hibernating individuals were regional migrants, i.e. they originated from populations located within the same δ2Hp isocline as their respective hibernacula. However, long-distance migrants originating from large catchment areas were documented for all hibernacula, demonstrating that long-distance migrants depend on intact hibernacula throughout Central Europe. This finding adds further to previous results from northern Germany [23], highlighting the importance of maintaining natural and possibly establishing artificial hibernacula as part of national and international conservation efforts.
In line with our prediction, we found a latitudinal gradient in the proportion of long-distance and regional migrants with higher proportions of long-distance migrants documented for hibernacula in northern regions than in southern areas. However, the majority of long-distance migrants consistently originated from isoclines adjacent to the sampling regions. This matches well with observations of bats banded in eastern Germany with a peak in migration distance around 500 km [15,23]. In general, the migration pattern observed in N. noctula neither completely corresponds to leap-frog nor to displacement migration. Instead, our results rather indicate that migratory individuals occur in all breeding colonies across Europe joining hibernacula at varying distances from summer areas (figure 1, Partial migration variant 2). Since noctule bats mate during migration and also in hibernacula, the combination of partial migration and highly variable migration distances promotes continuous genetic mixing of populations that are separated during the breeding season and likely contributes substantially to the panmictic genepool described for European noctule bats [35].
Past studies proposed differences in covered migration distances between sexes for N. noctula, with females travelling longer distances than males [12,18,36]. As expected, our isotopic data provide further evidence for differential migration in noctule bats over large geographical scales. Possibly, female noctules travel further north to benefit from high insect biomass production at northern latitudes during the period of high energetic requirements, i.e. pregnancy and lactation, whereas males remain in the area where they mate with females during migration and hibernation.
The observation of lower δ2Hf values for regional females compared to those of regional males suggests a similar differential migration pattern on the smaller, regional scale. Naturally, differential migration must not be limited to sex-related effects but likely involves differences based on age and reproductive status as well. These are important questions for improving conservation efforts, which need to be addressed in future research. Although migratory females clearly outnumbered males, considerable numbers of males were identified as migrants in some hibernacula, demonstrating that males may migrate long distances as well. However, because male noctules are hypothesized to disperse primarily in early life and to remain sedentary thereafter [37], the recorded male long-distance migrants could represent freshly dispersed individuals when hibernating for the first time.
In North America, migratory bats exhibit differential migratory behaviours as well (Lasionycteris noctivagans; [38], Lasiurus cinereus, and L. borealis [39]). Yet, it is difficult to compare migration patterns between continents, because information on wintering areas or hibernacula is sketchy for North America [40]. Thus, it is impossible to infer the catchment area and the level of partial migration over a larger geographical range for North American bats. However, based on isotopic data, it is apparent that both North American and European bats engage in continental-wide migration (this study [39,41,42]).
The isoscape origin approach used here to study migration patterns in noctule bats underlies a couple of assumptions which are discussed in detail in the section on assumptions and potential biases of the isoscape origin approach (see electronic supplementary material).
(b). Repeatability of individual migratory behaviour
Recently, ecosystems change dramatically due to a variety of anthropogenic factors, such as urbanization, agriculture, and climate change. Flexibility in individual migration strategies could constitute a powerful tool to cope with rapidly changing conditions and thus has the potential to enhance both resistance and resilience of bat populations. Our study is the first to test the repeatability of migration strategies in individually marked hibernating bats over several years. In contrast to our expectation, we found that individual migratory behaviours are highly specialized and show little variability over time with the vast majority of individuals either migrating each year or displaying signs of residency. Overall, only 14% of studied individuals showed variability in migration strategy over time, possibly indicating that migratory flexibility on the individual level may be limited to a small proportion of individuals within a given population. Individuals that migrated consistently were mostly females, providing further evidence for female-biased migration and female philopatry in noctule bats (e.g. [43,44]). By contrast, males are hypothesized to remain in a small geographically confined area for most of their life [37]. However, 30% of animals showing variability in migratory behaviour over time were males, demonstrating that a small portion of males may occasionally conduct long-distance migrations.
Moreover, our data demonstrate site fidelity of noctule bats towards hibernacula—whatever the distance to reach them—which poses interesting questions for future research on how juvenile bats learn where suitable hibernation sites are located and at what point in life they establish a tradition to hibernate at specific locations.
(c). Morphological correlates of migration
As predicted, we observed a negative correlation between forearm length and δ2Hf values in noctule bats, suggesting that migratory individuals had longer forearms than individuals with a more regional origin; a pattern observed in several bird species [45,46]. Additionally, we detected the sex-specific effects of δ2Hf values on body condition. While males seem to be in better body condition if they remain close to their hibernation area year round, females appear to benefit from migrating to northern summering areas. These findings add directly to our previous results from isoscape origin models providing strong support for female-biased migration in N. noctula. Further, we tested whether the detected sex-specific response of body condition to migration could be linked to the development of body condition over the course of hibernation (see electronic supplementary material for analyses). We found no indication for a sex-specific impact of hibernation on body condition. In general, amount of energy spent during hibernation seems to be directly linked to the initial body condition in autumn, with animals entering hibernation in good body condition using more energy than animals with less energy reserves. In line with this, Dechmann et al. [47] found that spring migration decision in female noctules is not correlated with the initial spring body condition or the fattening period, which modulates departure decisions in birds, but rather linked to environmental conditions optimal for migration. Reasons for the observed sex-related differences in migratory tendency and ‘migratory benefit’ in terms of body condition gain may therefore rather be found in the territorial autumn behaviours of males [20,48].
5. Conclusion
Using a stable isotope approach, we revealed patterns of partial and differential migration in the common noctule bat, N. noctula, across large parts of Europe, uncovering a latitudinal gradient in migratory activity with highest migration pressures on northern populations and providing evidence for female-biased migration. We showed that a considerable fraction of noctule bats is faithful both to their wintering as well as summer habitats and that phenotypic migratory behaviours—even in a partially migratory species—appear to be more invariable than expected.
Our findings call for careful management of known hibernacula of migratory bat species, because individuals are loyal to specific hibernacula and because the geographical catchment area of a given hibernacula is large.
Supplementary Material
Acknowledgements
We thank Anja Luckner, Karin Grassow, Doris Fichte, and Yvonne Klaar from the IZW stable isotope laboratory for isotope analyses. We thank Alexandre Courtiol for his highly constructive comments that substantially improved the manuscript. We are indebted to Andres Beck, Martin Biedermann, Inken Karst, Angelika Meschede, and Wigbert Schorcht for their support during fieldwork. L.S.L. was supported by a Landesgraduierten-Fellowship (Berlin).
Ethics
Work was conducted under the following permits issued by the relevant national or federal authorities: 120/2013 and FIBL1/12 for Switzerland, 86.44-12-0299/20155, 10315/12 and 4440-236-NF/001-2008 for Germany, DOPog-4201-04A-1/2002 for Poland, and 35601-35/2010-6 for Slovenia.
Data accessibility
The dataset supporting this article is available in the electronic supplementary material.
Authors' contributions
L.S.L. and C.C.V. conceived the ideas and designed methodology; L.S.L., T.T., U.H., A.P.-L., F.B., M.C., D.K.N.D., K.K., P.P., Ma.S., Mi.S., U.Z., and C.C.V. collected the data; L.S.L. and S.K.S. analysed the data; L.S.L. led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Competing interests
We have no competing interests.
Funding
L.S.L. was supported by a Landesgraduierten-Fellowship (Berlin).
References
- 1.Dingle H, Drake VA. 2007. What is migration? Bioscience 57, 113–121. ( 10.1641/B570206) [DOI] [Google Scholar]
- 2.Dingle H. 2014. Migration: the biology of life on the move. Oxford, UK: Oxford University Press. [Google Scholar]
- 3.Berthold P. 1984. The control of partial migration in birds: a review. Ring 10, 253–265. [Google Scholar]
- 4.Chapman BB, Brönmark C, Nilsson JÅ, Hansson LA. 2011. The ecology and evolution of partial migration. Oikos 120, 1764–1775. ( 10.1111/j.1600-0706.2011.20131.x) [DOI] [Google Scholar]
- 5.Mysterud A, Loe LE, Zimmermann B, Bischof R, Veiberg V, Meisingset E. 2011. Partial migration in expanding red deer populations at northern latitudes: a role for density dependence? Oikos 120, 1817–1825. [Google Scholar]
- 6.Terrill SB, Able KP. 1988. Bird migration terminology. The Auk 105, 205–206. [Google Scholar]
- 7.Cristol DA, Baker MB, Carbone C. 1999. Differential migration revisited. In Current ornithology, vol. 15 (eds Nolan V, Ketterson ED, Thompson CF), pp. 33–37. Boston, MA: Springer. [Google Scholar]
- 8.Marchetti K, Price T, Richman A. 1995. Correlates of wing morphology with foraging behaviour and migration distance in the genus Phylloscopus. J. Avian Biol. 26, 177–181. ( 10.2307/3677316) [DOI] [Google Scholar]
- 9.Maggini I, Spina F, Voigt CC, Ferri A, Bairlein F. 2013. Differential migration and body condition in Northern Wheatears (Oenanthe oenanthe) at a Mediterranean spring stopover site. J. Ornithol. 154, 321–328. ( 10.1007/s10336-012-0896-1) [DOI] [Google Scholar]
- 10.Newton I. 2012. Obligate and facultative migration in birds: ecological aspects. J. Ornithol. 153, 171–180. ( 10.1007/s10336-011-0765-3) [DOI] [Google Scholar]
- 11.Fudickar AM, Schmidt A, Hau M, Quetting M, Partecke J. 2013. Female-biased obligate strategies in a partially migratory population. J. Anim. Ecol. 82, 863–871. ( 10.1111/1365-2656.12052) [DOI] [PubMed] [Google Scholar]
- 12.Strelkov P. 1969. Migratory and stationary bats (Chiroptera) of the European part of the Soviet-Union. Acta Zool. Cracoviensia 14, 393–439. [Google Scholar]
- 13.Rojas-Martínez A, Valiente-Banuet A, Del Coro Arizmendi M, Alcántara-Eguren A, Arita HT. 1999. Seasonal distribution of the long-nosed bat (Leptonycteris curasoae) in North America: does a generalized migration pattern really exist? J. Biogeogr. 26, 1065–1077. ( 10.1046/j.1365-2699.1999.00354.x) [DOI] [Google Scholar]
- 14.Fleming TH, Eby P. 2003. Ecology of bat migration. In Bat ecology (eds Kunz TH, Fenton B), pp. 156–208. Chicago, IL: University of Chicago Press. [Google Scholar]
- 15.Steffens R, Zöphel U, Brockmann D. 2004. 40 jahre fledermausmarkierungszentrale dresden – methodische hinweise und ergebnisübersicht. Dresden, Germany: Sächsisches Landesamt für Umwelt und Geologie. [Google Scholar]
- 16.Hutterer R, Ivanova T, Meyer-Cords C, Rodrigues L. 2005. Bat migrations in Europe: a review of banding data and literature. Bonn, Germany: Federal Agency for Nature Conservation. [Google Scholar]
- 17.Dietz C, von Helversen O, Nill D. 2009. Handbook of the bats of Europe and northwest Africa. London, UK: A & C Books. [Google Scholar]
- 18.Lehnert LS, Kramer-Schadt S, Schönborn S, Lindecke O, Niermann I, Voigt CC. 2014. Wind farm facilities in Germany kill noctule bats from near and far. PLoS ONE 9, e103106 ( 10.1371/journal.pone.0103106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Heerdt P, Sluiter J. 1965. Notes on the distribution and behaviour of the noctule bat (Nyctalus noctula) in the Netherlands. Mammalia 29, 463–477. ( 10.1515/mamm.1965.29.4.463) [DOI] [Google Scholar]
- 20.Sluiter JW, van Heerdt PF. 1966. Seasonal habits of the noctule bat (Nyctalus noctula). Arch. Neerlandaises Zool. 16, 423–439. ( 10.1163/036551666X00011) [DOI] [Google Scholar]
- 21.Voigt CC, Lehnert LS, Petersons G, Adorf F, Bach L. 2015. Wildlife and renewable energy: German politics cross migratory bats. Eur. J. Wildl. Res. 61, 213–219. ( 10.1007/s10344-015-0903-y) [DOI] [Google Scholar]
- 22.Rydell J, Bach L, Dubourg-Savage MJ, Green M, Rodriguez L, Hedenström A. 2010. Bat mortality at wind turbines in north-western Europe. Acta. Chiropterol. 12, 261–274. ( 10.3161/150811010X537846) [DOI] [Google Scholar]
- 23.Voigt CC, et al. 2014. The trans-boundary importance of artificial bat hibernacula in managed European forests. Biodivers. Conserv. 23, 617–631. ( 10.1007/s10531-014-0620-y) [DOI] [Google Scholar]
- 24.Meschede A, Schorcht W, Karst I, Biedermann M, Fuchs D, Bontadina F. 2017. Wanderrouten der Fledermäuse. BfN-Skripten 453, 236 ( 10.19217/skr453) [DOI] [Google Scholar]
- 25.Brodersen J, Nilsson PA, Hansson LA, Skov C, Brönmark C. 2008. Condition-dependent individual decision-making determines cyprinid partial migration. Ecology 89, 1195–1200. ( 10.1890/07-1318.1) [DOI] [PubMed] [Google Scholar]
- 26.Popa-Lisseanu AG, et al. 2012. A triple isotope approach to predict breeding origins of European bats. PLoS ONE 7, e30388 ( 10.1371/journal.pone.0030388) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cryan PM, Bogan MA, Rye RO, Landis GP, Kester CL. 2004. Stable hydrogen isotope analysis of bat hair as evidence for seasonal molt and long-distance migration. J. Mammal. 85, 995–1001. ( 10.1644/BRG-202) [DOI] [Google Scholar]
- 28.Hobson KA. 1999. Tracing origins and migration of wildlife using stable isotopes: a review. Oecologia 120, 314–326. ( 10.1007/s004420050865) [DOI] [PubMed] [Google Scholar]
- 29.R Core Team. 2017. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; https://www.R-project.org/. [Google Scholar]
- 30.Courtiol A, Rousset F, Kramer-Schadt S. 2016. Isoscape computation and inference of spatial origins using mixed models. R package version 0.4-1.
- 31. IAEA/WMO 2017. Global Network of Isotopes in Precipitation. The GNIP Database. Accessible at: http://www.iaea.org/water .
- 32.Csorba G, Hutson AM. 2016. Nyctalus noctula. The IUCN Red List of Threatened Species 2016: e.T14920A22015682.
- 33.Zaccarelli N, Mancinelli G, Bolnick DI. 2013. RInSp: an R package for the analysis of individual specialisation in resource use. Meth. Ecol. Evol. 4, 1018–1023. ( 10.1111/2041-210X.12079) [DOI] [Google Scholar]
- 34.Roughgarden J. 1974. Niche width: biogeographic patterns among Anolis lizard populations. Am. Nat. 108, 429–442. ( 10.1086/282924) [DOI] [Google Scholar]
- 35.Petit E, Mayer F. 1999. Male dispersal in the noctule bat (Nyctalus noctula): where are the limits? Proc. R. Soc. Lond. B 266, 1717–1722. ( 10.1098/rspb.1999.0837) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petit E, Mayer F. 2000. A population genetic analysis of migration: the case of the noctule bat (Nyctalus noctula). Mol. Ecol. 9, 683–690. ( 10.1046/j.1365-294x.2000.00896.x) [DOI] [PubMed] [Google Scholar]
- 37.Petit E, Balloux F, Goudet J. 2001. Sex-biased dispersal in a migratory bat: a characterization using sex-specific demographic parameters. Evolution 55, 635–640. ( 10.1554/0014-3820(2001)055%5B0635:SBDIAM%5D2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 38.Fraser EE, Brooks D, Longstaffe FJ. 2017. Stable isotope investigation of the migratory behavior of silver-haired bats (Lasionycteris noctivagans) in eastern North America. J. Mammal. 98, 1225–1235. [Google Scholar]
- 39.Pylant CL, Nelson DM, Fitzpatrick MC, Gates EJ, Keller SR. 2016. Geographic origins and population genetics of bats killed at wind-energy facilities. Ecol. Appl. 26, 1381–1395. ( 10.1890/15-0541) [DOI] [PubMed] [Google Scholar]
- 40.Weller TJ, Castle KT, Liechti F, Hein CD, Schirmacher MR, Cryan PM. 2016. First direct evidence of long-distance seasonal movements and hibernation in a migratory bat. Sci. Rep. 6, 34585 ( 10.1038/srep34585) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cryan PM, Stricker CA, Wunder MB. 2014. Continental-scale, seasonal movements of a heterothermic migratory tree bat. Ecol. Appl. 24, 602–616. ( 10.1890/13-0752.1) [DOI] [PubMed] [Google Scholar]
- 42.Baerwald EF, Patterson WP, Barclay RMR. 2014. Origins and migratory patterns of bats killed by wind turbines in southern Alberta: evidence from stable isotopes. Ecosphere 5, 118 ( 10.1890/ES13-00380.1) [DOI] [Google Scholar]
- 43.Bels L. 1952. Fifteen years of bat banding in the Netherlands. vol. 5, pp. 1–99. Limburg, The Netherlands: Publicaties van het Natuurhistorisch Genootschap. [Google Scholar]
- 44.Heise G. 1989. Ergebnisse reproduktionsbiologischer Untersuchungen am Abendsegler (Nyctalus noctula) in der Umgebung von Prenzlau/ Uckermark. Nyctalus 3, 17–32. [Google Scholar]
- 45.Chandler CR, Mulvihill RS. 1990. Wing-shape variation and differential timing of migration in dark-eyed juncos. Condor 92, 54–61. ( 10.2307/1368382) [DOI] [Google Scholar]
- 46.Lockwood R, Swaddle JP, Rayner JMV. 1998. Avian wingtip shape reconsidered: wingtip shape indices and morphological adaptations to migration. J. Avian Biol. 29, 273–292. ( 10.2307/3677110) [DOI] [Google Scholar]
- 47.Dechmann DKN, Wikelski M, Ellis-Soto D, Safi K, Teague O'Mara M. 2017. Determinants of spring migration departure decision in a bat. Biol. Lett. 13, 20170395 ( 10.1098/rsbl.2017.0395) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gebhard J, Bogdanowicz W. 2004. Nyctalus noctula (Schreber, 1774) – Grosser Abendsegler. In Handbuch der säugetiere europas. Band 4/II: fledetiere (chiroptera) II (eds Niethammer J, Krapp F), pp. 607–694. Wiebelsheim: Aula-Verlag. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset supporting this article is available in the electronic supplementary material.