Abstract
Individuals with low empathy often show reduced attention towards social stimuli. A limitation of this literature is the lack of empirical work that has explicitly characterized how this relationship manifests itself over time. We investigate this issue by analysing data from two large eye-tracking datasets (total n = 176). Via growth-curve analysis, we demonstrate that self-reported empathy (as measured by the empathy quotient—EQ) predicts the temporal evolution of gaze behaviour under conditions where social and non-social stimuli compete for attention. In both datasets, we found that EQ not only predicted a global increase in social attention, but predicted a different temporal profile of social attention. Specifically, we detected a reliable effect of empathy on gaze towards social images after prolonged viewing. An analysis of switch latencies revealed that low-EQ observers switched gaze away from an initially fixated social image more frequently and at earlier latencies than high-EQ observers. Our analyses demonstrate that modelling these temporal components of gaze signals may reveal useful behavioural phenotypes. The explanatory power of this approach may provide enhanced biomarkers for conditions marked by deficits in empathy-related processes.
Keywords: eye-tracking, empathy, social attention
1. Introduction
To enable successful interactions with the environment, organisms must preferentially attend to socially significant stimuli. Failure to engage with conspecifics can result in exclusion and status loss, which are significant and recurrent fitness threats [1]. Moreover, attending to social stimuli allows the accumulation of strategically beneficial information such as the physical strength of a potential rival, the social standing of a potential ally or the genetic fitness of a potential mate [2]. In humans, such ‘social attention’ is also crucial for the development of communicative skills such as language acquisition and emotion recognition [3].
Empathy has been defined as the drive to identify with another person's emotions and thoughts, and to respond to these with an appropriate emotion [4]. In order to identify with another's emotions and respond appropriately, it is essential to attend to socially relevant cues such as bodily postures and facial expressions—which provide important information for decoding the emotional states of other people [5,6]. Social attention can therefore be conceptualized as an essential precursor to an empathic response. Support for this view has come primarily from case-control eye-tracking studies, which have demonstrated that individuals with deficits in some empathy related processes also show deficits in social attention. For instance, a recent meta-analysis revealed robust evidence that autism spectrum conditions (ASC) are associated with a reduction in social attention that generalizes across a wide range of tasks and stimulus conditions [7]. Influential case-control eye-tracking studies have indicated that individuals with ASC exhibit reduced attention to biological relative to non-biological motion patterns [8] and exhibit a preference to direct gaze towards geometric patterns when they compete with videos of social interactions [9]. However, other studies have called into question whether social attention differences are meaningfully related to the aetiology and maintenance of ASC [10,11]. The heterogeneity in reported outcomes is possibly due to the heterogeneous nature of ASC and the small sample sizes resulting from the practical issues associated with case-control designs. In this context, it is surprising that there is almost no literature that has attempted to model individual rather than group variation in social attention in the neurotypical population. One recent study has demonstrated that trait empathy is associated with a gaze bias towards social rewards in the neurotypical population [12]. Although this observation indicates that social attention is generally reduced in individuals with low empathy, the features of gaze behaviour underlying this reduction remain fundamentally unclear.
The output of a typical eye-tracking experiment is a continuous stream of spatial coordinates that define the location of an observer's gaze over time. To describe individual/ group differences in social attention, this time series is typically collapsed into the total gaze duration towards areas of interest (AOIs) containing social and non-social stimuli [7]. While total gaze duration is an intuitive and easily interpretable metric, it necessarily involves the removal of informative components of the data contained within the temporal domain. Such an approach may therefore fail in describing more subtle differences between individuals that describe the dynamic nature of social attention. Although some previous studies of social attention have considered the temporal origin of group differences via divergence analyses [13–15] none have provided or tested a quantitative model of the entire time series. To our knowledge, no existing study has provided an explicit model of the temporal structure of social attention and tested predictions about individual-level social gaze behaviour over time.
The motivation for investigating individual differences in the temporal structure of social attention is not purely data driven. At the theoretical level, prioritized perception of socially relevant signals is one of the most important functions of the visual system. As such, there is a major explanatory burden associated with identifying the features of gaze behaviour underlying individual variation in this phenomenon. Neurocognitive theories propose that social attention is mediated by neural circuits that transduce sensory information about conspecifics and translate that information into value signals that bias the spatial allocation of gaze over time [16]. In order to more fully appreciate what drives humans to attend to social aspects of the world, one must investigate the individual characteristics that influence this inherently dynamic process. By extension, this research effort may have the corollary of informing explanatory models of disordered social attention. Moreover, influential models propose that attention involves at least two distinct components of initial ‘orienting’ to and subsequent ‘maintaining’ of engagement with stimuli [17]. In global eye-tracking metrics, these two processes are conflated—total gaze duration towards social stimuli could reflect some combination of both the orienting and maintaining mechanisms. Delineating these mechanisms requires explicitly modelling the temporal components of the gaze signal. In general, we may expect empathy to primarily influence gaze behaviour some time after stimulus presentation because arriving at an empathic response may require sampling many relevant cues from a scene. We may need to attend to multiple subjects in the scene, determine their event roles, recognize their facial expressions/bodily postures and integrate this information over time before an empathic response is triggered. This idea is consistent with the recent observation that although empathy is predictive of gaze bias towards social images after prolonged viewing, it does not predict the initial saccadic deviation towards social images in a ‘global effect’ paradigm [12].
In the context of the preceding discussion, there is a clear lack of empirical work that has attempted to model the temporal structure of social attention and its relationship with individual social trait characteristics such as empathy. In this study, our goals were to (i) characterize the extent of gaze bias towards social stimuli in a large sample of observers, (ii) model the time course of this social bias and (iii) determine how empathy modulates the time course of the social bias. We report data from two large eye-tracking datasets, with a combined total of 176 observers.
2. Dataset 1
(a). Method
(i). Participants
Ninety-nine participants (58 females, mean age = 23, s.d. = 5) were recruited from in and around the University of Reading. Ethical approval for the study was obtained from the Research Ethics Committee of the University of Reading (Ethics ID: 2012/070/BC) and all participants provided informed consent. All participants had normal or corrected to normal vision. All participants except one female completed the empathy quotient (EQ) [18], a reliable, behaviourally validated measure of trait empathy. The mean EQ score was 44.21 (s.d. = 11.27), and the scores ranged from 25 to 73. This distribution of scores closely resembles that previously observed in large-scale surveys of the neurotypical population (e.g. [19]: n = 190, mean = 44.5, s.d. = 10.7).
(ii). Stimuli
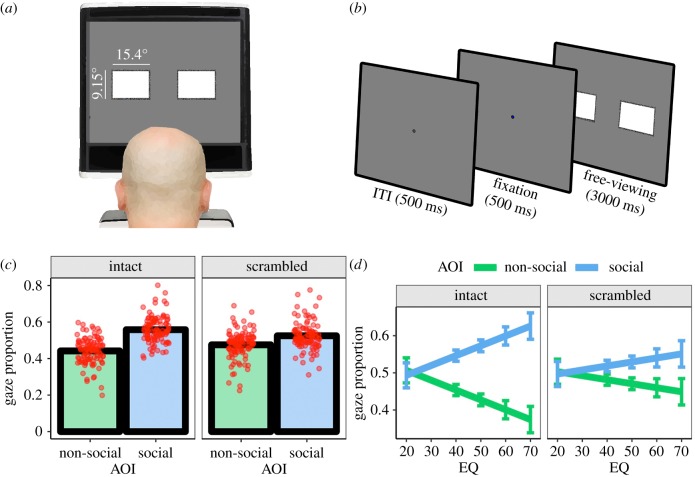
Forty pairs of social and non-social reward images were taken from the International Affective Picture System (18 pairs [20]) and downloaded from publicly available creative commons licensed images databases such as Flickr (22 pairs). All images were the same as used in [12], in which social reward images included one or more humans (e.g. happy individuals) while non-social reward images included rewarding non-social content (e.g. food, scenery and money—see electronic supplementary material S1). All stimuli in the experiment subtended 15.4 × 9.15 degrees of visual angle (DVA), and pairs were separated by 5.29 DVA (figure 1b).
Figure 1.
(a) Schematic of experimental set-up and (b) trial sequence. (c) Gaze proportion as a function of AOI and stimulus type. Red points indicate individual data. (d) Gaze proportion as a function of AOI, stimulus type and EQ. Error bars are ±1 s.e.m. (Online version in colour.)
To reduce the influence of extraneous sensory and affective differences between image pairs, all stimulus pairs were matched as closely as possible in terms of low level properties (e.g. luminance, contrast, saliency) as well as perceived valence and arousal—see electronic supplementary material S1. In addition, to further characterize the influence of low-level confounds, we presented two stimulus types. All image pairs were manipulated via randomly rearranging 10 × 10 pixel grids to create a set of ‘scrambled’ images in addition to the intact images. The logic of this manipulation is that if simple low-level variability between image pairs drives a gaze bias towards social images, we would expect to find a social bias of similar magnitude for both the intact and scrambled stimulus types. By contrast, if social bias is genuinely driven by the semantic content of the images, we would expect social bias to be substantially reduced for scrambled stimuli.
(iii). Procedure
Observers were seated 50 cm in front of a Tobii T60 eye-tracker with an inbuilt 1280 × 1024 pixel resolution monitor (60 hz refresh rate) and sampling rate of 60 Hz (figure 1a). Stimuli were presented using E-Prime 2.0 (Psychology Software Tools, PA, USA [21]). Following a 5-point calibration, participants completed the free-viewing task: Observers were informed that they would be presented with pairs of images side by side for 3 s, and that they were free to look wherever they liked during this period. Figure 1b depicts the trial sequence: observers were presented with a fixation cross for 500 ms, followed by a pair of the social and non-social stimuli for 3000 ms. To maintain engagement with the task, the colour of the fixation cross changed from black to blue on 10% of trials. The participant was asked to report these changes via button press as rapidly as possible. Observers completed 80 trials in total (40 image pairs, 2 stimulus types).
(b). Results
(i). Aggregated social bias
Data reduction was performed via the ‘eyetrackingR’ package, implemented in the R programming language [22]. The display coordinates occupied by the social and non-social images on each trial were defined as areas of interest (AOIs). We first analysed the data by aggregating across the time dimension. To this end, we reduced the raw gaze data for each participant into the proportion of trial time that gaze was directed into the social AOI and non-social AOI. This data was submitted to a general linear model with AOI (social, non-social) and stimulus type (intact, scrambled) as fixed effects. Reported significance tests of model coefficients were conducted via likelihood ratio tests of nested models containing the coefficients versus those without them. There was a main effect of AOI, indicating gaze bias towards social images χ12 = 104.02, p < 0.001. Moreover, the predicted interaction between AOI and stimulus type was detected χ12 = 18.92, p < 0.001 (figure 1c). The bias for social images was larger in the intact condition (β = 0.12) than scrambled condition (β = 0.05). Adding EQ to the model revealed a 3 way interaction between AOI, stimulus type and EQ χ12 = 5.90, p = 0.020. Higher EQ was associated with a larger social bias for intact stimuli than scrambled stimuli (figure 1d).
(ii). Time course of social bias
Having analysed the aggregated data expressed as total gaze duration, we next aimed to estimate a parsimonious model that described the time course of social bias across participants. For each observer, we first removed trials for which gaze failed to record for more than 60% of a trial (16% of the data). Next, we reduced each observer's gaze data into the proportion of gaze within the social and non-social AOI in each 100 ms time bin from the start to end of the trial. We then removed data from the first 100 ms time bin, since it contained 3 s.d. less than the mean number of valid samples captured within all time bins. No association was detected between EQ and the number of remaining data points when this cleaning strategy was applied r (96) = −0.019, p = 0.851.
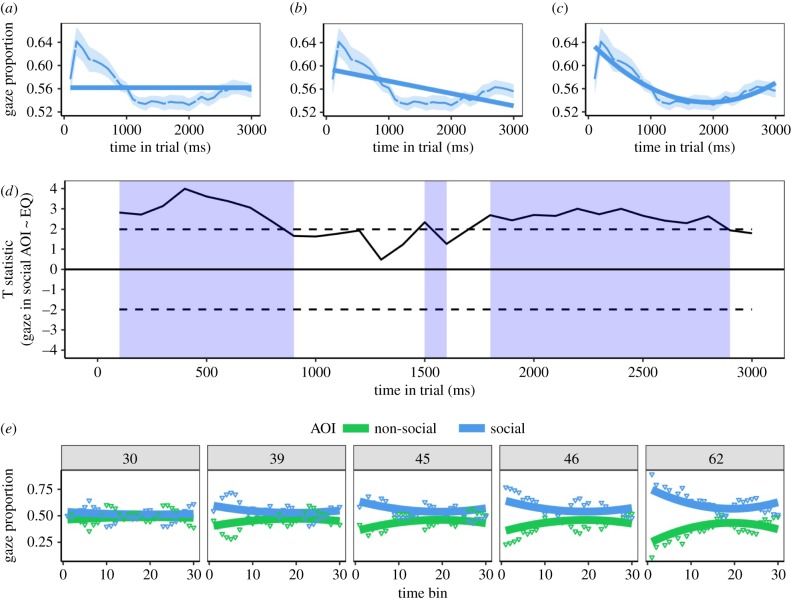
Figure 2a depicts the time course of gaze proportion into the social AOI for intact stimuli. This gaze bias towards social images is not time invariant (figure 2a), nor is its time course well described by a linear function (figure 2b). The global pattern is an initial bias towards the social AOI that peaks within the first 500 ms, followed by a nonlinear decline and a partial recovery towards the end of the trial. To model these nonlinear components of the time course, we proceeded via forward selection and tested the performance of models that included higher-order time regressors [23]. To protect against overfitting, we tested the generalization performance of each model, using standard leave one out (LOO) cross-validation procedures (see electronic supplementary material S2 and S3). Once linear and quadratic time regressors were added, the addition of higher order terms failed to reduce residuals or improve LOO performance, suggesting that more complex models were prone to overfitting. Therefore, a model with AOI and linear and quadratic time regressors as fixed effects (AIC = −6365.5) was retained as our global model of the time course of the social bias (figure 2c).
Figure 2.
(a) The time series fitted to the gaze proportion into the social AOI with only AOI as a fixed effect (no effect of time). (b) A fit to the same data with AOI and a linear time regressor as fixed effects. (c) The data fit with AOI and linear and quadratic time regressors. (d) t-statistics for the test that EQ is a linear predictor of gaze proportion into the social AOI within each 100 ms time bin. Shaded areas demarcate the time bins wherein the statistic reaches the (uncorrected) threshold for rejecting the null hypothesis. (e) Predictions of the fully interactive model for five observers. The panel headers indicate the observer's EQ score. Solid lines are model predictions, points are the empirical data. (Online version in colour.)
(iii). Effect of empathy on time course of social bias
Having modelled the time course of the social bias pooled across participants, we next attempted to model variation at the individual level. We first tested whether empathy modulates the time course of the social bias by defining EQ as a predictor of proportion of gaze in the social AOI within each 100 ms time bin. An effect of EQ as a predictor of gaze into the social AOI was detected within 3 ‘clusters’ of contiguous time bins (figure 2d, see electronic supplementary material S4 for a rationale for defining clusters). These were located (i) at 100–900 ms, (ii) at 1500–1600 ms, (iii) at 1800–2900 ms. Given the multiple tests associated with this analysis, our type 1 error rate may have reached unacceptable levels. Therefore, to protect against false positives, we performed a bootstrapped cluster-based permutation analysis (electronic supplementary material S4) akin to that typically applied to electroencephalogram data [24]. After this correction was applied, there was no detectable effect in the second cluster (p = 0.316), whereas the chances of obtaining the summed statistics observed in the first and last cluster under the null hypothesis were estimated to be at p = 0.003 and p = 0.002 respectively.
With this temporal influence of empathy established, we next proceeded to test models that added EQ as a fixed effect to our initial global model of the time course (electronic supplementary material S5). We first specified a reduced interactive model, which constrained EQ to interact only with AOI but not the time regressors. This led to improved model fit χ22 = 337.47, p < 0.001, consistent with the previously observed generalized increase in social bias associated with high EQ. Next we specified a fully interactive model, which removed this constraint and allowed EQ to additionally interact with the time regressors. This further improved on the reduced interactive model χ42 = 72.70, p < 0.001. To aid interpretation of this model, its predictions are plotted with the empirical data for five observers (figure 2e), whose EQ is ordered from left to right (low to high). The model predicts that EQ is associated with a generalized increase in gaze bias towards the social AOI (i.e. the vertical offset between the blue and green lines), but that this effect is particularly pronounced at the start and end of the trial. Given the complexity of this fully interactive model, we again protected against overfitting via another LOO analysis, which confirmed that this model had the superior performance (electronic supplementary material S5).
In good agreement with the results of our cluster-based analysis, this confirms that EQ is not only associated with a generalized increase in social bias, but also with a different temporal profile of social bias. Inspection of figure 2e reveals that EQ predicts an initial increase in social attention, but also a more sustained component that maintains social attention at the later portions of the trial.
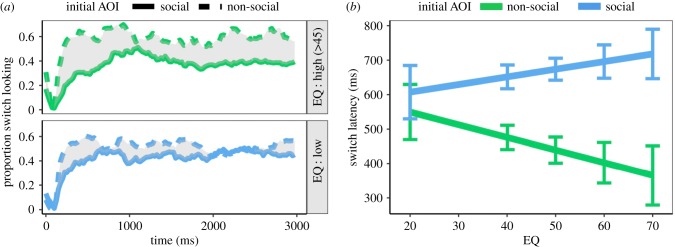
One plausible mechanism for this sustained component is that, after being initially fixated, social images hold attention for longer durations in high-empathy individuals than low-empathy individuals. To test this possibility, we split trials according to the AOI that was initially fixated and analysed the latency at which observers switched their gaze to the alternate AOI. We reasoned that if empathy was associated with sustained attention on social images, this would be manifested in an interactive effect of EQ and initial AOI on gaze switch latency. Figure 3a depicts the proportion of observers who switched AOI as a function of the initial AOI, EQ (median split for visualization) and time. Inspection of this figure reveals that low-EQ individuals switched from the social AOI more frequently and at earlier latencies than high-EQ individuals. The predicted interaction between EQ and initial AOI on switch latency was detected χ12 = 4.56, p = 0.030. Higher EQ was associated with later switching from the social AOI relative to the non-social AOI (figure 3b).
Figure 3.
(a) Proportion of observers who switched to the alternate AOI as a function of initial AOI, EQ (median split) and time. (b) Switch latency as a function of initial AOI and EQ. Error bars are ±1 s.e.m. (Online version in colour.)
3. Dataset 2
Our analyses of the first dataset indicate a robust effect of empathy on the time course of social attention. To further validate our initial findings, we next tested their generalization performance via a re-analysis of an existing, independent dataset [12].
(a). Method
(i). Participants
Seventy-seven participants (42 females; mean age = 21 years, s.d. = 3 years) drawn from in and around the University of Reading campus completed the FV task. All participants had normal or corrected to normal vision. Sixty-eight (38 female) participants completed the online EQ questionnaire. The study was approved by the University of Reading Research Ethics Committee (Ethics ID: 2010/86/BC).
(ii). Stimuli
The images and image pairings were the same as those described for Dataset 1.
(iii). Procedure
The only procedural differences from those described in Dataset 1 were as follows. Participants were seated at 100 cm from a 1600 × 1200 pixel resolution colour monitor (75 hz refresh rate). Eye movements were recorded via a video based eye-tracker with a sampling rate of 500 hz (Eyelink 2, SR research). Stimuli were presented via Experiment Builder software [25]. The presentation duration of stimuli in this task was 5000 ms and stimuli subtended 5.59 × 4.19 DVA.
(b). Results
(i). Aggregated social bias
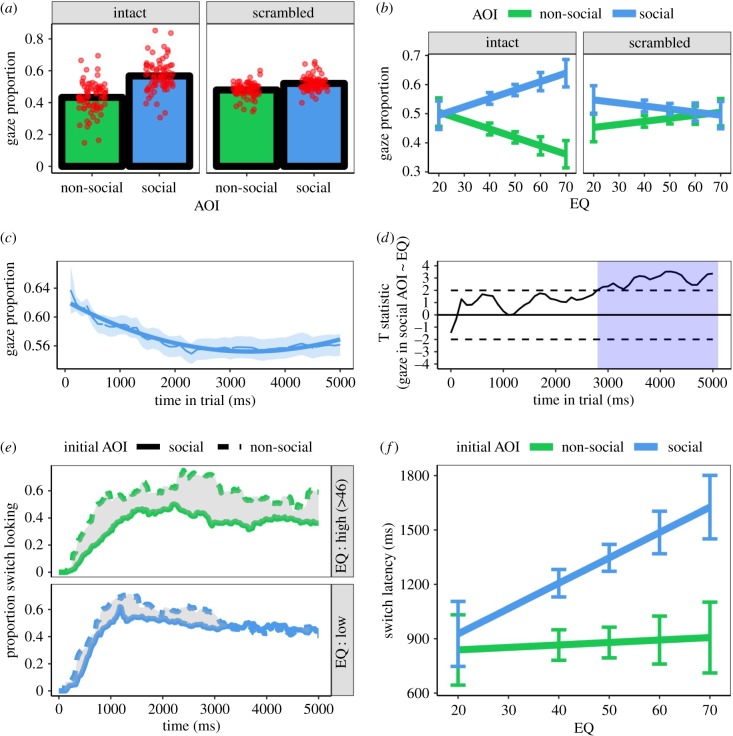
Inspection of figure 4 reveals a pattern of results that very closely mirror those obtained from Dataset 1. There was again the same main effect of AOI χ12 = 91.40, p < 0.001 and interaction between AOI and stimulus type χ12 = 28.61, p < 0.001 (figure 4a). The bias for social images was similarly larger in the intact condition (β = 0.13) than scrambled condition (β = 0.04). Adding EQ to the model revealed the same three-way interaction between AOI, stimulus type and EQ χ12 = 18.21, p < 0.001. Higher EQ was associated with a larger social bias for intact stimuli, but not scrambled stimuli (figure 4b).
Figure 4.
(a) Gaze proportion as a function of AOI and stimulus type. Red points indicate individual data. (b) Gaze proportion as a function of AOI, stimulus type and EQ. Error bars are ±1 s.e.m. (c) The fit to gaze proportion time series with AOI and a linear and quadratic time regressors as fixed effects. Data are shown for the social AOI. (d) t-statistics for the test that EQ is a linear predictor of gaze proportion into the social AOI within each 100 ms time bin. Shaded areas demarcate the time bins wherein the statistic reaches the (uncorrected) threshold for rejecting the null hypothesis. (e) Proportion of observers who switched to the alternate AOI as a function of initial AOI and EQ (median split). (f) Switch latency as a function of initial AOI and EQ. Error bars are ±1 s.e.m. (Online version in colour.)
(ii). Time course of social bias
We used the same data reduction strategy as reported for Dataset 1. We removed 2.85% trials due to trackloss and again removed data from the first 100 ms time bin. No association was detected between EQ and the number of remaining data points when this cleaning strategy was applied r (67) = −0.003, p = 0.981. The forward selection strategy revealed that a model involving AOI and linear and quadratic time regressors as fixed effects (figure 4c) again provided the best fit to the data (AIC −9639.3) and had the best generalization performance (see electronic supplementary material S6).
(iii). Effect of empathy
An effect of EQ as a predictor of social bias was detected within a cluster from 2800 to 5000 ms (corrected p = 0.009—figure 4d). We again tested models that added EQ as a fixed effect to our initial model of the global data. The reduced interactive model again improved model fit χ22 = 335.98, p < 0.001. Moreover, a fully interactive model further improved on the reduced interactive model χ42 = 85.14, p < 0.001. EQ was primarily predictive of social bias towards the end of the trial (figure 4d).
An analysis of switch latencies did not detect an interaction between initial AOI and EQ χ12 = 3.52, p = 0.060, but the effect was similar in magnitude and direction to that observed in Dataset 1. Higher EQ was again associated with later switching to the social AOI relative to from the non-social AOI (figure 4e,f).
4. Discussion
In this study our major novel contributions were as follows: We (i) provide an explicit model of the time course of social attention, (ii) determine how the parameters of this model are modulated by social trait characteristics of the observer, (iii) test this model by making quantitative predictions about the allocation of an individual's gaze over time. Across two large datasets, we found a number of similar findings. (i) Observers exhibit a robust gaze bias towards social images. (ii) EQ is reliably associated with an increase in this bias. (iii) This effect of EQ is not time invariant—a model that allowed empathy to interact with the temporal components of the gaze bias provided a superior fit to a model that assumed a time-invariant effect of empathy. Specifically, empathy was found to reliably maintain gaze bias towards social images after prolonged viewing. (iv) Higher EQ was associated with less frequent and later switching from an initially fixated social image.
At the most fundamental level, our finding that gaze behaviour is predicted by the social trait characteristics of the observer emphasizes that the mechanisms underlying social attention are deeply enmeshed with other aspects of social cognition. The dynamic influence of empathy on gaze behaviour suggests that empathy is not a passive affective resonance with the emotions of others and that wider contextual influences play feed-forward roles in how emotions are perceived and experienced. This fits with neurocognitive theories of empathy, which propose that empathy is implemented by a network of recursively connected cortical and subcortical sites [26]. It also fits well with multi-stage models of empathy, which propose that prolonged attention to social stimuli reflects a form of evidence gathering so that appropriate empathic responses can be generated [27,28].
Our findings appear consistent with recent pharmacological work, which indicates that administration of oxytocin (associated with the experience of empathy in humans and mesolimbic dopaminergic activity involved in responding to rewards) predicts maintained periods of eye-contact in macaque monkeys [14]. We speculate the similarity of these findings with our own reflect some common mechanism that promotes prolonged perceptual selection of socially relevant inputs. Computational models of alternative forced choice behaviour have been proposed that explicitly relate gaze behaviour to value coding. The ‘gaze cascade model’ proposes that gaze and value coding mutually interact, resulting in an increased gaze towards preferred stimuli over time [29]. A consistent observation from both of our datasets is that trait empathy is better able to predict gaze towards social rewards towards the end of the trial. One potential interpretation of this observation is that trait empathy is related to enhanced motivational salience of social stimuli. By extension, we speculate that the individual differences in the temporal evolution of eye-movement behaviour observed in our study reflects some online behavioural correlate of the value-coding process. This inference relies on electrophysiological studies that show value-coding is a dynamic process, and requires accumulation of evidence over time [30]. This interpretation of empathy being related to the value coding of social rewards is also consistent with the observation that higher empathy is associated with greater reward-related striatal activation in response to social reward stimuli [31]. Our free-viewing task, of course, did not require observers to make an explicit choice between two stimuli. Recent computational modelling of binary choice behaviour indicates that impressive predictions of choice behaviour can be generated by models that incorporate gaze behaviour and the reward value of competing stimuli [32]. In this context, an interesting question concerns whether empathy similarly predicts different trajectories of social attention and different gaze cascade effects in choice-based paradigms.
In interpreting our findings, it is important to acknowledge that gaze behaviour in response to complex rewarding scenes is likely to reflect the output of many dissociable and fundamental processes. As such, the pattern of results we found could also be driven by some combination of component processes found to vary as a function of empathy. This may include individual differences in gaze perception [33], expression recognition [34], temporal integration [15] and a precedence of local over global processing [35]. Our data cannot clarify the relative contribution of these factors. Moreover, gaze behaviour is strongly determined by low-level properties, such as luminance contrast and spatial frequency profile. Although we attempted to protect against these issues with our matching procedures and use of scrambled control stimuli, our stimuli are still not immune to these issues. However, no study involving complex, naturalistic visual stimuli is completely resistant to these potential confounds.
In the absence of longitudinal data, a claim about the directionality of the causal relationship between empathy and social attention observed here is clearly over-reaching. Based on the available developmental literature, however, there are sensible grounds for proposing that some aspects of social attention precede empathy. Newborns exhibit robust orienting responses to conspecific stimuli (particularly faces) [36], whereas the cognitive components of empathy (such as theory of mind) emerge several years into development [37]. In this context, our study could motivate well-controlled developmental studies that track the temporal structure of social attention across development and its shared trajectory with the development of empathic abilities.
Our findings have several important implications for the design of future studies. We observed that empathy can take effect on behaviour several seconds after stimulus onset. Spontaneous mimicry, related to certain components of empathy [38], can also take effect several seconds after stimuli onset (e.g. in response to reward [39]). Findings like these may question the sensitivity of methods that rely on much briefer stimulus exposures, such as visual probe paradigms [40–42] in detecting differences between groups that vary in empathic traits. There is widespread enthusiasm for the idea that electrophysiological methods with high temporal resolution may further clarify the temporal brain dynamics of empathy [43,44] and distinguish between competing explanatory models. Based on the findings reported in this paper, we are additionally enthusiastic about the prospect of paradigms that employ concurrent recording of both EEG and gaze data. Capitalizing on the high temporal resolution shared by these methods may lead to theoretical advancement by providing insight into the time course of the neural signatures underlying empathy and their behavioural correlates. Motivated accounts of empathy suggest that observers may dynamically increase or decrease attention to social cues to regulate their emotional responses [28]. Paradigms that concurrently monitor gaze allocation and autonomic arousal over time could explicitly test the predictions of such models.
In general, our data demonstrate that considering the temporal structure of gaze signals may provide impetus towards enhanced behavioural phenotypes for conditions marked by deficits in one or more empathy-related processes (ASC, psychopathy, bipolar disorder, schizophrenia [45–47]). More broadly, follow-up experimentation of this variety can also help us answer the more fundamental question: what features of gaze behaviour differentiate between individuals with and without these conditions? Failing to capitalize on the high-dimensional, time-varying nature of gaze signals necessarily entails restricting the information available for answering this question.
Supplementary Material
Acknowledgements
The authors wish to acknowledge the help from Loredana Canzano, Charlotte Whiteford, Natalie Kkeli, Violetta Mandreka and Kara Dennis in stimulus preparation and data collection.
Ethics
Ethical approval for these studies was obtained from the University of Reading local ethics committee. Dataset 1 (Ethics ID: 2012/070/BC) Dataset 2: (Ethics ID: 2010/86/BC). Informed consent was obtained from all participants by the experimenters.
Data accessibility
The datasets supporting this article are available as part of the electronic supplementary material.
Authors' contributions
N.H. carried out the statistical analysis and wrote the initial draft of the paper. B.C., E.M. and A.H. conceived of the study design, coordinated data collection and provided modifications to the initial manuscript draft. All authors declare that they have no conflicting or competing interests in relation to this article.
Competing interests
All authors declare that they have no competing interests that influence the presentation of this manuscript.
Funding
B.C. was supported by Leverhulme Trust (PLP-2015-329) and Medical Research Council (G1100359/1, MR/P023894/1) during this work. A.H. was supported by an ESRC MRC Interdisciplinary PhD studentship.
References
- 1.Boyer P, Bergstrom B. 2011. Threat-detection in child development: an evolutionary perspective. Neurosci. Biobehav. Rev. 35, 1034–1041. ( 10.1016/j.neubiorev.2010.08.010) [DOI] [PubMed] [Google Scholar]
- 2.Hayden BY, Parikh PC, Deaner RO, Platt ML. 2007. Economic principles motivating social attention in humans. Proc. R. Soc. B 274, 1751–1756. ( 10.1098/rspb.2007.0368) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chevallier C, Kohls G, Troiani V, Brodkin ES, Schultz RT. 2012. The social motivation theory of autism. Trends Cogn. Sci. 16, 231–239. ( 10.1016/j.tics.2012.02.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams RB Jr, Adams RB, Ambady N, Shimojo S, Nakayama K (eds). 2011. The science of social vision. Oxford, UK: Oxford University Press. [Google Scholar]
- 5.Hafri A, Trueswell JC, Strickland B. 2018. Encoding of event roles from visual scenes is rapid, spontaneous, and interacts with higher-level visual processing. Cognition 175, 36–52. ( 10.1016/j.cognition.2018.02.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein JT, Shepherd SV, Platt ML. 2009. Social attention and the brain. Curr. Biol. 19, R958–R962. ( 10.1016/j.cub.2009.08.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chita-Tegmark M. 2016. Social attention in ASD: a review and meta-analysis of eye-tracking studies. Res. Dev. Disabil. 48, 79–93. ( 10.1016/j.ridd.2015.10.011) [DOI] [PubMed] [Google Scholar]
- 8.Klin A, Lin DJ, Gorrindo P, Ramsay G, Jones W. 2009. Two-year-olds with autism orient to non-social contingencies rather than biological motion. Nature 459, 257–261. ( 10.1038/nature07868) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pierce K, Marinero S, Hazin R, McKenna B, Barnes CC, Malige A. 2016. Eye tracking reveals abnormal visual preference for geometric images as an early biomarker of an autism spectrum disorder subtype associated with increased symptom severity. Biol. Psychiatry 79, 657–666. ( 10.1016/j.biopsych.2015.03.032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischer J, Koldewyn K, Jiang YV, Kanwisher N. 2014. Unimpaired attentional disengagement and social orienting in children with autism. Clin. Psychol. Sci. 2, 214–223. ( 10.1177/2167702613496242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah P, Gaule A, Bird G, Cook R. 2013. Robust orienting to protofacial stimuli in autism. Curr. Biol. 23, R1087–R1088. ( 10.1016/j.cub.2013.10.034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chakrabarti B, Haffey A, Canzano L, Taylor CP, McSorley E. 2017. Individual differences in responsivity to social rewards: insights from two eye-tracking tasks. PLoS ONE 12, e0185146 ( 10.1371/journal.pone.0185146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sasson N, Tsuchiya N, Hurley R, Couture SM, Penn DL, Adolphs R, Piven J. 2007. Orienting to social stimuli differentiates social cognitive impairment in autism and schizophrenia. Neuropsychologia 45, 2580–2588. ( 10.1016/j.neuropsychologia.2007.03.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dal Monte O, Piva M, Anderson KM, Tringides M, Holmes AJ, Chang SWC. 2017. Oxytocin under opioid antagonism leads to supralinear enhancement of social attention. Proc. Natl Acad. Sci. USA 114, 5247–5252. ( 10.1073/pnas.1702725114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakano T, Tanaka K, Endo Y, Yamane Y, Yamamoto T, Nakano Y, Ohta H, Kato N, Kitazawa S. 2010. Atypical gaze patterns in children and adults with autism spectrum disorders dissociated from developmental changes in gaze behaviour. Proc. R. Soc. B 277, 2935–2943. ( 10.1098/rspb.2010.0587) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang SWC, Brent LJN, Adams GK, Klein JT, Pearson JM, Watson KK, Platt ML. 2013. Neuroethology of primate social behavior. Proc. Natl Acad. Sci. USA 110(Suppl. 2), 10 387–10 394. ( 10.1073/pnas.1301213110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Posner MI, Snyder CR, Davidson BJ. 1980. Attention and the detection of signals. J. Exp. Psychol. 109, 160–174. ( 10.1037/0096-3445.109.2.160) [DOI] [PubMed] [Google Scholar]
- 18.Lawrence EJ, Shaw P, Baker D, Baron-Cohen S, David AS. 2004. Measuring empathy: reliability and validity of the Empathy Quotient. Psychol. Med. 34, 911–919. ( 10.1017/S0033291703001624) [DOI] [PubMed] [Google Scholar]
- 19.Baron-Cohen S, Wheelwright S. 2004. The empathy quotient: an investigation of adults with Asperger syndrome or high functioning autism, and normal sex differences. J. Autism Dev. Disord. 34, 163–175. ( 10.1023/B:JADD.0000022607.19833.00) [DOI] [PubMed] [Google Scholar]
- 20.Unifesp. Undated. International Affective Picture System (IAPS). See https://www2.unifesp.br/dpsicobio/adap/instructions.pdf.
- 21.Psychology Software Tools. 2016. E-Prime 2.0. See http://www.pstnet.com.
- 22.eyetrackingR. Undated. eyetrackingR. See http://www.eyetracking-r.com/.
- 23.Mirman D, Dixon JA, Magnuson JS. 2008. Statistical and computational models of the visual world paradigm: growth curves and individual differences. J. Mem. Lang. 59, 475–494. ( 10.1016/j.jml.2007.11.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maris E, Oostenveld R. 2007. Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods 164, 177–190. ( 10.1016/j.jneumeth.2007.03.024) [DOI] [PubMed] [Google Scholar]
- 25.SR Research. 2011 Experiment Builder 1.10.165 [computer software]. Mississauga, Canada: SR Research Ltd.
- 26.Decety J. 2010. The neurodevelopment of empathy in humans. Dev. Neurosci. 32, 257–267. ( 10.1159/000317771) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bird G, Viding E. 2014. The self to other model of empathy: providing a new framework for understanding empathy impairments in psychopathy, autism, and alexithymia. Neurosci. Biobehav. Rev. 47, 520–532. ( 10.1016/j.neubiorev.2014.09.021) [DOI] [PubMed] [Google Scholar]
- 28.Zaki J. 2014. Empathy: a motivated account. Psychol. Bull. 140, 1608–1647. ( 10.1037/a0037679) [DOI] [PubMed] [Google Scholar]
- 29.Shimojo S, Simion C, Shimojo E, Scheier C. 2003. Gaze bias both reflects and influences preference. Nat. Neurosci. 6, 1317 ( 10.1038/nn1150) [DOI] [PubMed] [Google Scholar]
- 30.Kim H, Sul JH, Huh N, Lee D, Jung MW. 2009. Role of striatum in updating values of chosen actions. J. Neurosci. 29, 14 701–14 712. ( 10.1523/JNEUROSCI.2728-09.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chakrabarti B, Bullmore E, Baron-Cohen S. 2006. Empathizing with basic emotions: common and discrete neural substrates. Soc. Neurosci. 1, 364–384. ( 10.1080/17470910601041317) [DOI] [PubMed] [Google Scholar]
- 32.Krajbich I, Armel C, Rangel A. 2010. Visual fixations and the computation and comparison of value in simple choice. Nat. Neurosci. 13, 1292–1298. ( 10.1038/nn.2635) [DOI] [PubMed] [Google Scholar]
- 33.Pantelis PC, Kennedy DP. 2017. Deconstructing atypical eye gaze perception in autism spectrum disorder. Sci. Rep. 7, 14990 ( 10.1038/s41598-017-14919-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harms MB, Martin A, Wallace GL. 2010. Facial emotion recognition in autism spectrum disorders: a review of behavioral and neuroimaging studies. Neuropsychol. Rev. 20, 290–322. ( 10.1007/s11065-010-9138-6) [DOI] [PubMed] [Google Scholar]
- 35.Dakin S, Frith U. 2005. Vagaries of visual perception in autism. Neuron 48, 497–507. ( 10.1016/j.neuron.2005.10.018) [DOI] [PubMed] [Google Scholar]
- 36.Johnson MH, Dziurawiec S, Ellis H, Morton J. 1991. Newborns’ preferential tracking of face-like stimuli and its subsequent decline. Cognition 40, 1–19. ( 10.1016/0010-0277(91)90045-6) [DOI] [PubMed] [Google Scholar]
- 37.Leslie AM, Friedman O, German TP. 2004. Core mechanisms in ‘theory of mind’. Trends Cogn. Sci. 8, 528–533. ( 10.1016/j.tics.2004.10.001) [DOI] [PubMed] [Google Scholar]
- 38.Pfeifer JH, Iacoboni M, Mazziotta JC, Dapretto M. 2008. Mirroring others’ emotions relates to empathy and interpersonal competence in children. Neuroimage 39, 2076–2085. ( 10.1016/j.neuroimage.2007.10.032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sims TB, Van Reekum CM, Johnstone T, Chakrabarti B.. 2012. How reward modulates mimicry: EMG evidence of greater facial mimicry of more rewarding happy faces. Psychophysiology 49, 998–1004. ( 10.1111/j.1469-8986.2012.01377.x) [DOI] [PubMed] [Google Scholar]
- 40.Zhao X, Zhang P, Fu L, Maes JHR. 2016. Attentional biases to faces expressing disgust in children with autism spectrum disorders: an exploratory study. Sci. Rep. 6, 19381 ( 10.1038/srep19381) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moore DJ, Heavey L, Reidy J. 2012. Attentional processing of faces in ASD: a Dot-Probe study. J. Autism Dev. Disord. 42, 2038–2045. ( 10.1007/s10803-012-1449-4) [DOI] [PubMed] [Google Scholar]
- 42.Quintana DS, et al. 2017. Dose-dependent social-cognitive effects of intranasal oxytocin delivered with novel Breath Powered device in adults with autism spectrum disorder: a randomized placebo-controlled double-blind crossover trial. Transl. Psychiatry 7, e1136 ( 10.1038/tp.2017.103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neumann DL, Westbury HR. 2011. The psychophysiological measurement of empathy. In Psychology of empathy (ed. DJ Scapaletti), pp. 119–142 New York, NY: Nova Science Publishers. [Google Scholar]
- 44.Suzuki Y, Galli L, Ikeda A, Itakura S, Kitazaki M. 2015. Measuring empathy for human and robot hand pain using electroencephalography. Sci. Rep. 5, 15924 ( 10.1038/srep15924) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Derntl B, Finkelmeyer A, Toygar TK, Hülsmann A, Schneider F, Falkenberg DI, Habel U. 2009. Generalized deficit in all core components of empathy in schizophrenia. Schizophr. Res. 108, 197–206. ( 10.1016/j.schres.2008.11.009) [DOI] [PubMed] [Google Scholar]
- 46.Jones AP, Happé FGE, Gilbert F, Burnett S, Viding E. 2010. Feeling, caring, knowing: different types of empathy deficit in boys with psychopathic tendencies and autism spectrum disorder: comparing empathy deficits in boys with psychopathic tendencies and ASD. J. Child Psychol. Psychiatry 51, 1188–1197. ( 10.1111/j.1469-7610.2010.02280.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shamay-Tsoory S, Harari H, Szepsenwol O, Levkovitz Y. 2009. Neuropsychological evidence of impaired cognitive empathy in euthymic bipolar disorder. J. Neuropsychiatry Clin. Neurosci. 21, 59–67. ( 10.1176/jnp.2009.21.1.59) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article are available as part of the electronic supplementary material.