Abstract
Observing friendly social interactions makes people feel good and, as a result, then act in an affiliative way towards others. Positive visual contagion of this kind is common in humans, but whether it occurs in non-human animals is unknown. We explored the impact on female Barbary macaques of observing grooming, a behaviour that physiological and behavioural studies indicate has a relaxing effect on the animals involved. We compared females' behaviour between two conditions: after observing conspecifics groom, and in a matched control period. We found that observing grooming was associated with reduced behavioural indicators of anxiety, suggesting that seeing others groom is, in itself, relaxing. Observing grooming was also associated with a shorter latency to becoming involved in a grooming bout (and higher likelihood both of initiating that bout and being the groomer rather than groomee), and with elevated rates of other affiliative behaviours. These results provide evidence for positive visual contagion; this phenomenon may contribute fundamentally to group cohesion not just in this species, but also in the many mammal and bird species where grooming occurs. Our study highlights the importance of exploring social behaviour beyond the level of the interacting individuals, within the broader social context where it occurs.
Keywords: audience, social, cooperation, primate, social network, eavesdrop
1. Introduction
Repeated interactions between individual animals underlie their social relationships, which in turn underpin species’ social structure [1]. Understanding how and why animals engage socially with each other is, as a result, a central goal of behavioural ecology [2]. Studies of animal social interactions typically focus only on the individuals immediately involved; such interactions do not usually occur in isolation, however, but rather in the presence of other group members. Seeing or hearing conspecifics interacting can alter the affective state and behaviour of these bystanders, leading to contagion—the spread of affect and behaviours from one individual to others in the group [3,4]. This phenomenon may have important impacts on individual animals, and more broadly at the level of their social networks, and such effects have been well studied in humans [5]. In non-human species, they are much less well understood, but there is increasing evidence that contagion—of negative and positive affective states and behaviours—occurs in a range of taxa.
Evidence that negative interactions of conspecifics lead to contagion among bystanders has been found in a range of taxa. In rats, Rattus norvegicus, behavioural, pharmacological and brain stimulation studies indicate that specific 22 kHz vocalizations given in aversive social interactions reflect underlying negative affective states [6], and individuals hearing these calls in an experimental setting showed behavioural indicators of anxiety, namely a reluctance to enter and explore an open arena [7]. In primates, there is behavioural and pharmacological evidence that self-directed behaviours such as scratching indicate affective state: decreases in these behaviours from normal levels reflect feelings of relaxation [8], while increases indicate anxiety [9,10]. In a number of primate species, e.g. hamadryas baboons, Papio hamadryas [11] and Japanese macaques, Macaca fuscata [12], it has been found that bystanders witnessing an aggressive interaction between other group members showed elevated levels of self-directed behaviours.
Studies exploring how positive interactions of conspecifics may lead to contagion in bystanders have focussed on a range of behaviours associated with positive affect, and their associated acoustic cues. For example, in common marmosets, Callithrix jacchus, playbacks of chirp calls given during affiliation led to an increase in rates of positive social behaviours [13], and in zoo-housed groups of chimpanzees, Pan troglodytes, the frequency of grooming behaviour was found to be positively related to the number of grooming-related vocalizations from a neighbouring group [14]. In kea parrots, Nestor notabilis, individuals hearing the playback of calls given in the context of social play showed an increase in likelihood of playing with conspecifics [15] and in rats, playbacks of ultrasonic calls given during play led to an increase in prosocial approach behaviour [16].
A notable gap in our knowledge relates to positive contagion through visual observation of conspecific interactions. This phenomenon is central to human social interactions [17–19]; seeing friendly interactions can make people feel positive emotions and, as a result, they then act in an affiliative way to others [20,21]. To our knowledge, only one study has explored such positive visual contagion beyond our own species, despite the fact that many group-living species rely heavily on vision to monitor conspecifics' behaviour [22–24]. In that study, Watson [25] found that laboratory-housed common marmosets shown videos of conspecifics grooming showed elevated rates of grooming, but did not show reduced levels of self-scratching as would be expected if they experienced a positive shift in affective state [8]. Moreover, prolonged exposure to videos led to an increase in self-scratching, suggesting that the video presentations were stressful; the increase in grooming associated with such presentations may consequently represent a behavioural coping strategy to alleviate such stress [26].
Here, we tested for evidence of positive visual contagion among Barbary macaques, Macaca sylvanus, by investigating their response to observing grooming interactions. Grooming occurs in a wide range of mammal and bird species [27–31] and there is evidence that this behaviour provides hedonic benefits, relaxing those involved [32]. Being groomed is associated with a reduced heart rate in pigtail macaques, Macaca nemestrina [33], rhesus macaques, Macaca mulatta [34] and Camargue horses, Equus caballus [35], a release of opioids in the blood in pigtail macaques [36], and lower rates of self-directed behaviour in long tailed macaques, Macaca fascicularis [37] and green woodhoopoes, Phoeniculus purpureus [31]. The giving of grooming has been found to be associated with reduced rates of self-directed behaviour in crested macaques, Macaca nigra [38] and green woodhoopoes [31], and with lower stress hormone levels in Barbary macaques [39]. In chimpanzees, grooming with a closely bonded social partner—regardless of the direction of grooming—is associated with an increase in peripheral oxytocin levels [40].
In this observational study of semi-free-ranging adult female Barbary macaques, we tested the hypothesis that observing grooming leads to positive contagion. Such contagion could result in positive changes in affective state, promote grooming, increase rates of other affiliative behaviour, or inhibit agonistic behaviour; we explored predictions related to each of these four possibilities. We predicted firstly that the observation of grooming would reduce bystanders’ rates of self-directed behaviour (prediction 1). We also predicted that observing grooming would reduce the time to bystanders' next grooming bout (prediction 2a), that levels of visual attention while observing grooming would be negatively related to the time to the next grooming bout (prediction 2b), and that observing grooming would increase the likelihood both of bystanders initiating grooming (prediction 2c) and of them being the groomer rather than groomee (prediction 2d). We predicted that observing grooming would increase bystanders’ rates of approaching other individuals (prediction 3a), the proportion of time they spent in close proximity to others (prediction 3b) and their rates of (non-grooming) affiliative behaviour (prediction 3c), but would reduce their rates of aggressive behaviour (prediction 4).
2. Methods
(a). Study site and animals
We conducted this study in the semi-free ranging population of Barbary macaques at Trentham Monkey Forest (Stoke-on-Trent, UK). Barbary macaques live in multi-male, multi-female groups in which grooming is a key social behaviour. Importantly, unlike many other primate species, Barbary macaques do not have grooming-specific vocalizations, and it is extremely rare for any vocal signals to be given during a grooming bout, so acoustic cues are unlikely to underpin any observed contagion effects. At Trentham, two groups of Barbary macaques range within a fenced 24 ha area of grassland, oak and cedar forest. Visitors to the park must stay on designated paths and are not allowed to touch or feed the animals. The macaques are provisioned with fruit, vegetables, pellets and cereals. Each monkey has an individual code tattooed on the inside of their thigh, allowing individual identification. Subjects of this study were 20 adult females, aged from 4 to 27 years old (mean ± s.d.: 12.8 ± 6.7 years old); females were chosen as subjects as they are involved in grooming more frequently than males. These animals lived in the same group, which comprised 68 individuals at the start of the study: 31 adult females (greater than 4 years of age), 22 adult males (greater than 4 years of age) and 15 individuals less than 4 years of age (nine females and six males). During the study period, three infants were born.
(b). Data collection
We conducted behavioural observations daily from 9.00 to 5.00 between 1 April and 15 June 2017. The procedure used to collect data was adapted from the well-established post-conflict/matched-control (PC-MC) method, first used by de Waal & Yoshihara [41] to study post-conflict behaviour. In the PC-MC method, observational data are collected on the behaviour of individually recognized animals during a defined period of time after they have been involved in a conflict (PC), and then compared to data collected for the same animal during an MC before which no conflict occurred. In our study, instead of collecting data following conflicts involving our focal animals, we did so after the start of their observation of a grooming bout.
We recorded these post-observing-grooming (POG) samples opportunistically, starting them either (i) when a grooming interaction started between two individuals, with one of our study subjects less than 7 m from the grooming dyad; or (ii) when a study subject moved to a distance less than 7 m from two individuals already involved in a grooming interaction. We decided on the maximum distance of 7 m between the focal individual and the grooming dyad following a brief pilot study. Bystanders frequently attended to grooming interactions over distances up to 7 m and while they sometimes appeared to look at more distant grooming bouts, we felt the accuracy of assessment of gaze direction reduced markedly beyond this distance.
For a POG to be used in the study, the grooming interaction observed by the study subject had to involve at least one adult individual (male or female) and to last at least 1 min after the start of the POG. In addition, the subject had to be awake, to look at the grooming dyad at least once, and to stay in a 7 m radius of them for at least 1 min. In order to assess the looking behaviour of the subject, where possible the observer stood such that the grooming animals were between them and the subject; thus the observer looked beyond the grooming pair to the subject, and a look was scored when the gaze of the subject was assessed to be directly at the grooming interaction. In a very small number of cases, such alignment was not possible, and here a look was scored when the orientation of the subject's head was judged to be directly towards the grooming animals.
For each POG, we recorded the length of the grooming bout (or the time until the focal animal moved away to greater than 7 m) and the number of times the subject looked directly at the grooming dyad. We followed the subject until the start of their next grooming interaction with another individual, which marked the end of the POG. For this next grooming interaction, we recorded whether the subject was groomer or groomee, and whether they did, or did not, initiate the bout (the subject was considered as initiator if they approached another individual and either started grooming them, or presented to be groomed by them). If no grooming bout involving the subject occurred within one hour of the start of the POG, data collection was stopped.
On the day following the POG, or as soon as was possible thereafter, we carried out MC observations, starting at about the same time of day (± 30 min), collecting the same type of data and for the same amount of time as the corresponding POG. We followed the focal individual for 10 min before the beginning of the MC to be sure they were not a bystander of a grooming interaction or themselves involved in grooming during this period; if they were, the MC was postponed to the next day. Similarly, if the subject was involved in an intense fight or conflict in this time, the MC was postponed. If, during the MC, the subject was located within 7 m of individuals involved in grooming and obvious visual attention towards the grooming dyad was detected, we abandoned the MC and started a new POG; two MCs were then subsequently collected in chronological order for the two POGs (i.e. the first MC was matched to the first POG). If no visual attention towards the grooming bout was detected, the MC continued until the end. If it was not possible to carry out an MC within two weeks after the date of a POG, we discarded that POG (mean interval between POG and MC was 4.11 days). We stopped data collection on those subjects who gave birth during the study period (n = 3) after the birth of their infant, and discarded any POGs which had been recorded before the birth but for which the MCs had not yet been collected.
During the POG and MC observation periods, we recorded all occurrences of self-directed behaviours (scratching, self-grooming, yawning and body shake) shown by the focal animal. Occurrences of self-directed behaviours had to be separated by a minimum of 5 s to be considered two separate events. We also noted the occurrence of any non-grooming affiliative behaviours (body contact, affiliative facial expression, embrace, affiliative touch, mount and co-feeding), and aggressive behaviours (bite, chase, contact aggression, mock hit, aggressive facial expressions, lunge and scream). We recorded all occurrences of subjects approaching another individual (to within 1 m). We used scan sampling to assess the proximity of the subject to adult males or females during POGs and MCs, noting every minute the presence of all such animals within 1 m and within 5 m of the focal individual (with distances estimated by eye).
Behavioural observations were recorded using an iPod Touch equipped with the application Animal Behaviour Pro© v.1.2 [42], with the exception of three observations which, owing to an iPod malfunction on one day, were recorded by voice onto mobile phone. Treated data are available in the electronic supplementary material, and all raw data files are deposited at https://figshare.com/articles/Berthier_and_Semple_-_raw_data_files/7029269.
(c). Data analyses
To ensure that in each POG-MC pair the duration of behavioural observation was the same, in cases where grooming involving the focal individual occurred earlier during an MC than during the corresponding POG, we reduced the length of the latter to the same length as the former. Following de Waal & Yoshihara [41], we then classified each POG-MC pair as: ‘attracted’ (when a grooming interaction involving the subject occurred during the POG period but not in the corresponding MC), ‘dispersed’ (when a grooming interaction involving the focal individual occurred before the end of the MC period; this includes POG-MC pairs for which no grooming interaction involving the focal occurred during the POG but one did occur during the MC), or ‘neutral’ (grooming did not happen in either the POG or MC; n = 7 in total, one from each of seven females). To avoid pseudoreplication, for each study animal we calculated an average of each behaviour (or proportion of attracted/dispersed pairs) across their POGs and their MCs, and used these individual-level matched pairs of data to test most of the study predictions, with paired t-tests used when difference scores were found (using the Shapiro–Wilk test) to be normally distributed, and Wilcoxon matched-pairs tests used when difference scores were not normally distributed. Statistical tests were two-tailed with alpha set at 0.05, and conducted in SPSS v.22. We tested predictions as follows.
-
(i)
Prediction 1: we used a paired t-test to determine whether rates of self-directed behaviours were lower in POGs than MCs.
-
(ii)
Prediction 2a: we used a Wilcoxon matched-pairs test to determine whether the proportion of ‘attracted’ POG/MC pairs was higher than that of ‘dispersed’ POG/MC pairs.
-
(iii)
Prediction 2b: we tested whether visual attention of subjects towards the grooming bouts observed in POGs was negatively related to time to the next grooming bout, following the method described by Carder & Semple [43] and Hohmann et al. [44]. First, for each subject with at least five POGs (n = 19), Spearman's rank correlations were carried out to assess the relationship between the number of times subjects looked towards the grooming bout, and the time to their next grooming interaction (the latter set at 60 min if no grooming occurred during a POG). We then used a one-sample t-test to test whether the mean of subjects' correlation coefficients was significantly lower than 0, as predicted if the overall pattern of relationships between visual attention and time to next grooming bout is negative.
-
(iv)
Prediction 2c: we used a paired t-test to determine whether the proportion of POGs for which the subjects were the initiator of the next grooming interaction was higher than the proportion for which other individuals were the initiator of the next grooming interaction. One female for which only one grooming interaction was observed in POGs was excluded from this analysis (all other females had at least four grooming interactions in total in POGs). We repeated this analysis for MCs, predicting here that proportions of grooming bouts initiated versus not initiated by subjects would not be different, reflecting an overall baseline pattern of animals initiating on average half of the grooming bouts they are involved in. In MCs, the next grooming bouts involving subjects were frequently not observed; thus, only those subjects for which at least three grooming interactions were seen were considered in these analyses (n = 10).
-
(v)
Prediction 2d: we used a paired t-test to determine whether the proportion of POGs for which the subjects were the groomer in the next grooming interaction was higher than the proportion in which they were the groomee (as above, the female for which only one grooming interaction was observed in POGs was excluded from analysis). For the corresponding analysis of MCs (for which we used a Wilcoxon matched-pairs test), as before subjects’ next grooming bouts were often not observed, and only subjects for which at least three grooming interactions were seen were considered (n = 11).
-
(vi)
Predictions 3a-3c: we used Wilcoxon matched-pairs tests to determine whether rates of approaching another individual (to within 1m) (prediction 3a), the proportion of time spent with at least one neighbour within 1 m, and within 5 m (prediction 3b), and rates of (non-grooming) affiliative behaviours (prediction 3c) were higher in POGs than MCs.
-
(vii)
Prediction 4: we used a Wilcoxon matched-pairs test to determine whether rates of aggressive behaviours were lower in POGs than MCs.
3. Results
In total, 154 POG/MC pairs were collected over the 20 adult females in this study (range: 2–10 pairs per female), representing a total of 82 h 7 min of observation. Each individual was followed for a mean of 4 h 27 min (range 2 h 6 min–7 h 44 min). For 15 out of 154 POGs (9.7% of the dataset), no grooming interaction involving the focal individual was observed after 1 h of observation; for seven of these (one from each of seven females) no grooming was seen also in the MC.
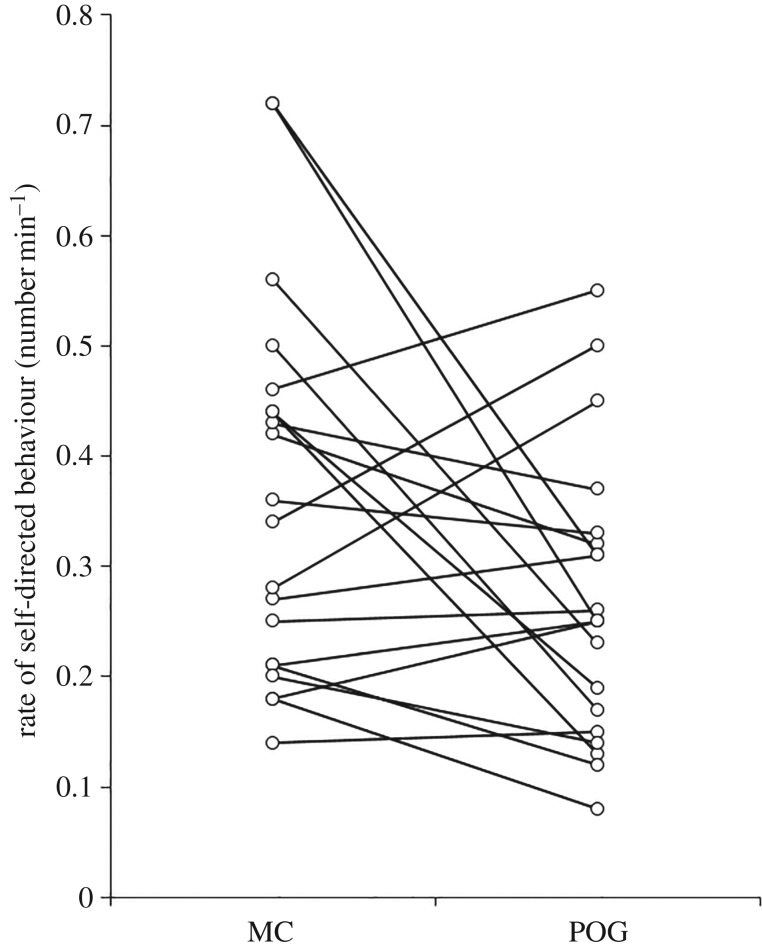
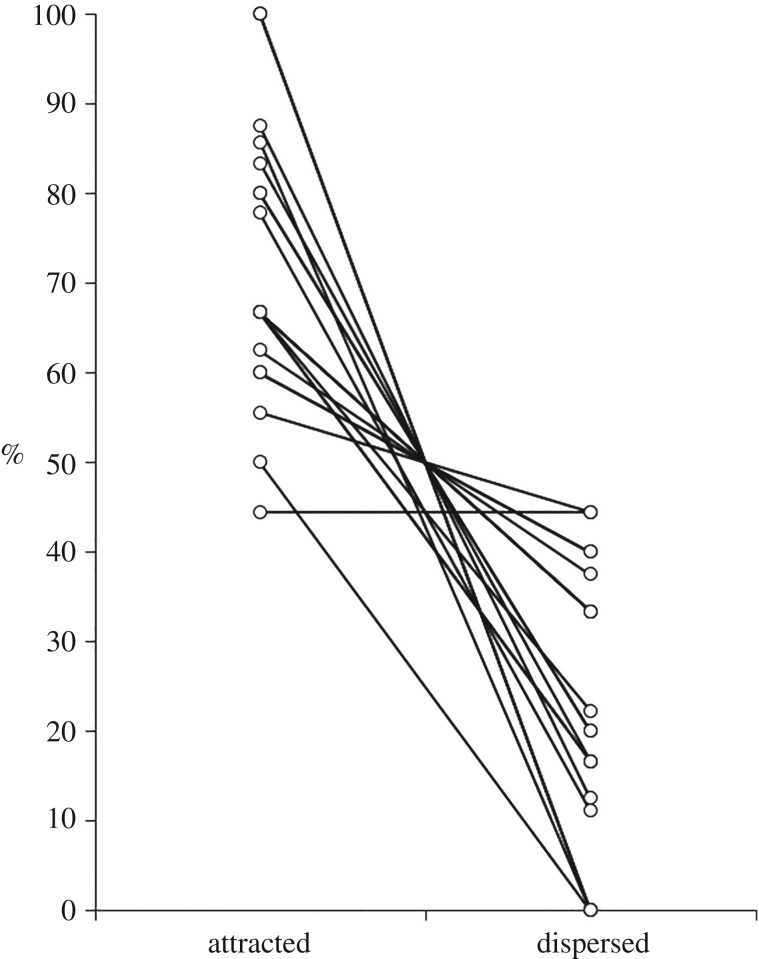
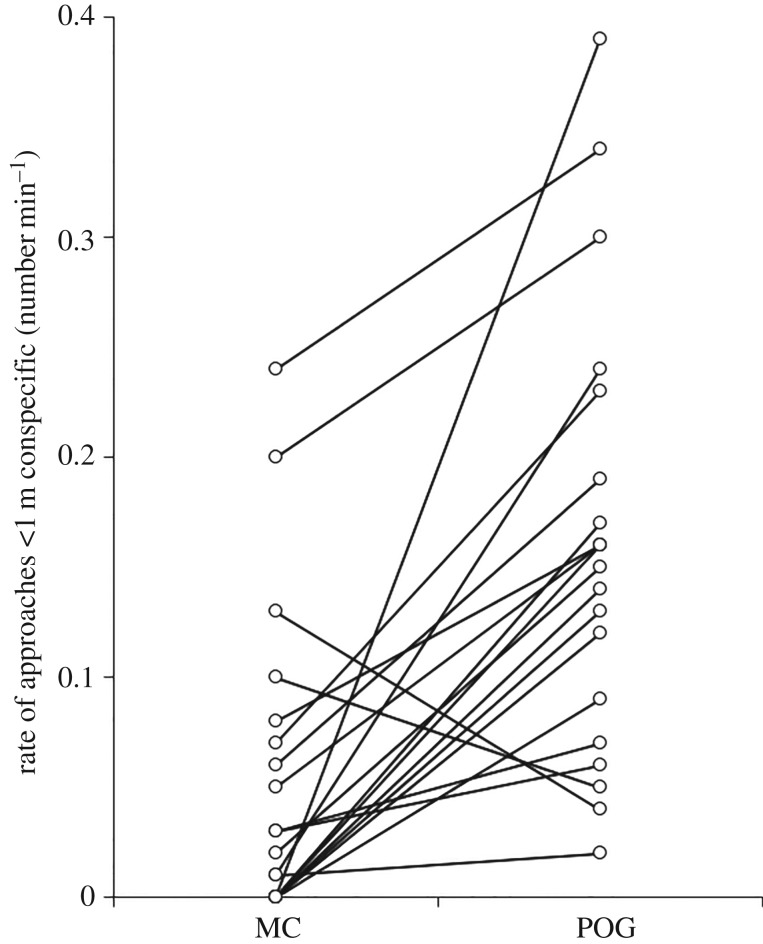
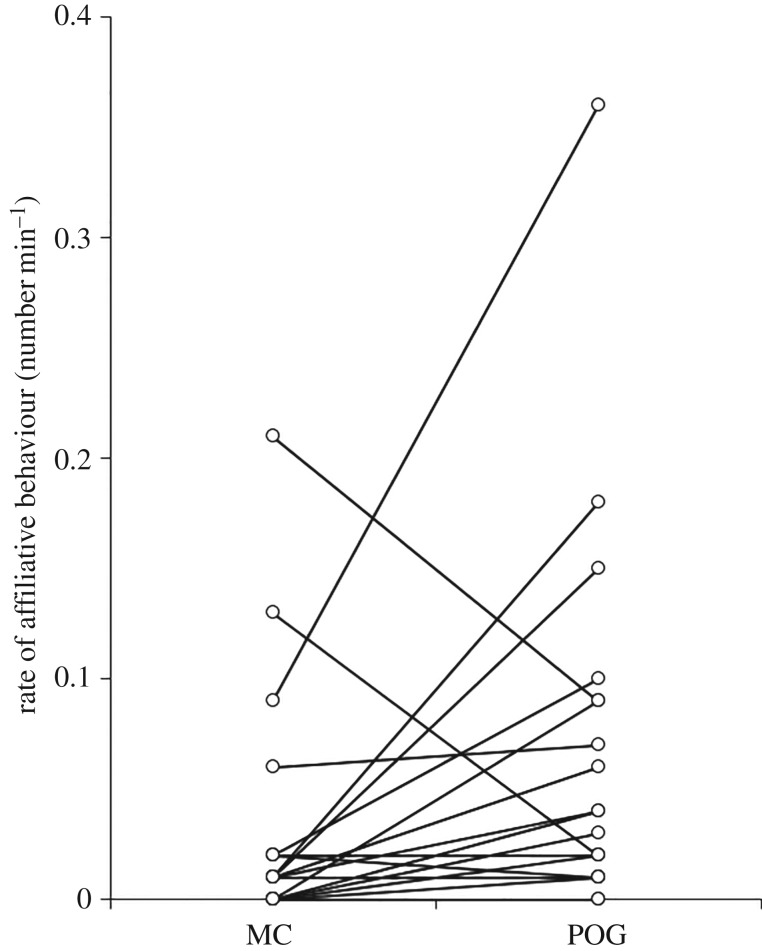
In support of prediction 1, rates of self-directed behaviours were lower during POGs than during MCs (paired t-test: t19 = −2.276, p = 0.035; figure 1). In support of predictions 2a and 2b, the average proportion of attracted POG/MC pairs was significantly higher than the average proportion of dispersed pairs (attracted: median = 0.67, range = 0.44–1.00; dispersed: median = 0.20, range = 0.00–0.44; Wilcoxon matched-pairs test: Z = −3.827, n = 20, p < 0.001; figure 2), and the rate of visual attention towards the grooming bout was negatively associated with the time to the next grooming interaction (one sample t-test: t18 = −2.226, p = 0.039). Supporting prediction 2c, in POGs the proportion of grooming interactions initiated by the focal individual was significantly higher than the proportion initiated by another individual (initiated by focal: mean = 0.62, range = 0.33–1.00; initiated by other: mean = 0.38, range = 0.00–0.67; paired t-test: t18 = 2.852, p = 0.011); by contrast, in MCs the proportion of grooming interactions initiated by the focal individual was not significantly different to the proportion of grooming interactions initiated by another individual (initiated by focal: mean = 0.57, range = 0.00–1.00; initiated by other: mean = 0.43, range = 0.00–1.00; paired t-test: t9 = 0.749, p = 0.473). Supporting prediction 2d, in POGs the proportion of grooming interactions for which the focal individual was the groomer was significantly higher than the proportion for which the focal individual was the groomee (groomer: mean = 0.72, range = 0.33–1.00; groomee: mean = 0.26, range = 0.00–0.67; paired t-test: t18 = 5.603, p < 0.001); in MCs, by contrast, the proportion of grooming interactions for which the focal individual was the groomer was not significantly different to the proportion for which the focal individual was the groomee (groomer: median = 0.60, range = 0.00–0.71; groomee: median = 0.33, range = 0.00–0.67; Wilcoxon matched-pairs test: Z = −1.429, n = 11, p = 0.153). Predictions 3a-3c were all supported: females approached other individuals more frequently during POGs than MCs (Wilcoxon matched-pairs test: Z = −3.530, n = 20, p < 0.001; figure 3), spent significantly more time with at least one conspecific in proximity during POGs than MCs (within 1 m—Wilcoxon matched-pairs test: Z = −1.972, n = 20, p = 0.049; within 5 m—Wilcoxon matched-pairs test: Z = −2.688, n = 20, p = 0.007), and were involved in significantly more (non-grooming) affiliative interactions during POGs than MCs (Wilcoxon matched-pairs test: Z = −2.112, n = 20, p = 0.035; figure 4). Finally, prediction 4 was not supported as females were not less aggressive in POGs compared to MCs (Wilcoxon matched-pairs tests: Z = −0.699, n = 20, p = 0.485).
Figure 1.
Rates of self-directed behaviour in post-observing-grooming (POG) samples and matched controls (MC). Lines join data points for individual females (n = 20).
Figure 2.
Proportion of ‘attracted’ and ‘dispersed’ post-observing-grooming (POG) and matched controls (MC) pairs. Lines join data points for individual females (n = 20). Note that as POG/MC pairs could also be ‘neutral’, values for individual females do not necessarily total 100%.
Figure 3.
Rates of approaching conspecifics in post-observing-grooming (POG) samples and matched controls (MC). Lines join data points for individual females (n = 20).
Figure 4.
Rates of (non-grooming) affiliative behaviour in post-observing-grooming (POG) samples and matched controls (MC). Lines join data points for individual females (n = 20).
4. Discussion
In this study of adult female Barbary macaques, we tested whether observing grooming—an affiliative behaviour that behavioural and physiological studies suggest has a relaxing effect on the animals involved—leads to positive contagion among bystanders. Our results indicate that seeing conspecifics groom was associated with a reduction in a behavioural indicator of anxiety among bystanders, suggesting that seeing others groom is, in itself, relaxing. In addition observation of grooming bouts was associated with increases in a range of affiliative behaviours, including grooming itself. These findings provide evidence from a non-human species that observing affiliative interactions of conspecifics can lead to positive contagion. This work further highlights the importance of exploring animal social behaviour not just at the level of the interacting individuals, but also within the broader social environment in which the behaviour occurs.
Female Barbary macaques showed lower rates of self-directed behaviours after observing others grooming than in corresponding control periods. This mirrors the decrease in self-directed behaviours from baseline levels seen in captive long-tailed macaques that had been given lorazepam [8,10], a drug which in healthy humans has a relaxing effect, leading to feelings of calmness [45]. Our results indicate, therefore, that seeing others grooming has a calming effect. This is at least suggestive of the occurrence of emotional contagion—sharing the emotional state of another [46]—but to provide strong evidence for this phenomenon, it would be necessary to assess simultaneously the emotional state of the grooming animals and bystanders, and to demonstrate a change in the emotional state of the latter towards the state of the former [47]. For this, measures of emotion that reflect valence as well as arousal should be used [47,48]; these could be provided by combining behavioural indices with non-invasive physiological measures, for example through remote assessment of heart rate [49] or quantification of urinary levels of oxytocin [40] or cortisol [50].
We also found evidence that grooming was itself contagious: subjects observing others grooming were quicker to become involved in a grooming bout themselves, and more likely to be both the initiator of this bout and the giver rather than the receiver of grooming. Moreover, the more frequently a bystander looked at a grooming interaction, the shorter was the time to their next grooming bout, suggesting that intensity of visual attention is an important factor in grooming contagion. Animals that had seen others grooming were also more likely to approach and to spend time in close proximity to conspecifics, and to engage with them in (non-grooming) affiliative behaviours. Taken together, these findings indicate that animals become more tolerant and prosocial after seeing others interacting in a positive way, perhaps as a result of reduced anxiety levels. Interestingly, rates of aggression did not appear to be impacted by observing grooming interactions. This suggests that contagion effects related to grooming may be valence-specific, i.e. they manifest themselves as increased rates of socio-positive behaviours, but not reduced rates of socio-negative behaviours.
Positive visual contagion of the kind for which we have provided evidence here is likely to play a key role in maintaining group cohesion [51]. In humans, it has been found that positive contagion influences work group dynamics, in particular, increasing levels of within-group cooperation [52]. Our study suggests that a similar phenomenon may arise in non-human animals, as a result of the impacts of observing others engaged in positive social interactions. The contagion of affiliation in general, and grooming, in particular, would be expected to strengthen social bonds and promote cooperation among group members. In primates, the giving of grooming leads not just to the reciprocation of grooming [53] but also to increased social tolerance and support in conflicts [54,55], access to infants [56] and mating opportunities [57]. Visual contagion related to grooming may therefore give rise to a multi-faceted ripple effect, extending throughout the social network of the group—from bystanders to their subsequent grooming partners, to the bystanders of those grooming interactions and beyond. Exploring the nature and reach of such chains of behavioural contagion will provide valuable new insights into the importance of visual contagion effects in shaping both within- and between-species differences in affiliative tendencies. Variation in the strength of such effects may, for example, drive differences in affiliation between populations of the same species, or within such populations over time. Moreover, interspecific differences in visual contagion may underpin variation in social style across species, with higher levels of positive contagion characterizing more tolerant societies.
It is important to consider the adaptive significance for bystanders of the positive visual contagion effects we document. Important benefits may arise from bystanders' consequent affiliative social interactions; in Barbary macaques, animals that groom others are more likely to be tolerated around valuable food resources and to receive support in agonistic encounters [55]. Additionally, the costs associated with grooming may be reduced if it occurs when group mates are also grooming. For example, the time cost of searching for a willing grooming partner, and/or the risk of receiving aggression from a potential partner, may be lower in such contexts as other animals in the group have demonstrated a readiness to engage in grooming at that particular time. The relative opportunity costs of grooming may also be reduced by engaging in this behaviour when others are doing so, as these individuals will also be incurring such costs, being similarly not able to exploit the alternative opportunities available.
Grooming is one of the most commonly studied social behaviours in animals, with data on patterns of grooming used to test predictions from a range of theoretical frameworks including reciprocal altruism, kin selection and biological markets (e.g. [58–62]). To date, such work has typically focussed on the animals directly involved in the interaction, with little attention paid to the ways that bystanders might influence—or be influenced by—grooming bouts. The impact of bystanders on grooming interactions has recently started to be explored, and evidence indicates that these individuals can have direct effects by intervening to disrupt ongoing grooming bouts [63], or indirect effects by their presence affecting grooming partner choice [64] or the nature of the grooming interaction [65,66]. Our study indicates the value of exploring now the other side of the coin—the impact of grooming interactions on bystanders. Furthermore, the evidence we present that observing grooming has an impact on bystanders raises an intriguing possibility, namely that grooming may have a signalling function, and that in some situations bystanders are not mere eavesdroppers but rather intended receivers. Theoretical and empirical studies to assess potential benefits to groomers of the impact of their behaviour on bystanders are needed to test this idea.
Overall, the findings of this study further highlight the importance of moving the analysis of animal social behaviour beyond the level of the interacting individuals, to take into account the broader social environment; in doing so, we feel there are a number of key avenues for future exploration. Firstly, it would be valuable to explore inter-individual variability in the extent to which observing affiliative interactions leads to positive contagion, and to investigate the biological correlates of such variation; key variables that have been linked previously to variation in affective response, and that might therefore be important here, include sex [67], age [68] and physiological parameters such as levels of circulating oxytocin [69]. Secondly, it would be interesting to investigate the factors—for example, the rank, identity of, or relatedness to, the animals being observed—that may mediate the occurrence or intensity of such contagion. Finally, it would be valuable to explore interspecific variation in this phenomenon to test, for example, whether propensity to positive contagion covaries with species’ social style (e.g. tolerant/despotic). Studies of these kinds are needed if we are to appreciate the role that positive visual contagion plays in the life of social animals.
Supplementary Material
Acknowledgements
We thank the staff of Trentham Monkey Forest for permission to conduct the research and for their generous assistance throughout the study. We thank Dr Nadine Beckmann, Dr Colette Berbesque, Dr Kirsten Bell, Prof. Fulvio d'Acquisto, Piotr Fedurek, Dr Lewis Halsey, Dr Harry Marshall, Dr Alan McElligott and Dr Caroline Ross for their valuable comments on an earlier version of this paper. We thank also Prof. Thomas Bugnyar and six anonymous reviewers for their thoughtful and detailed reviews of the submitted manuscript.
Ethics
Ethical approval was provided by the University of Roehampton. Permission to conduct the research was granted by the Trentham Monkey Forest and the Department of Life Sciences at the University of Roehampton. The study animals were fully habituated to visitors and researchers, and all data were collected using behavioural observations only. The observer attempted to keep a minimum distance of 5 m from all monkeys at all times, and physical and direct eye contact with study animals was strictly avoided.
Data accessibility
Treated data are available in the electronic supplementary material; all raw data files are deposited at https://figshare.com/articles/Berthier_and_Semple_-_raw_data_files/7029269.
Authors' contributions
J.M.B. and S.S. designed research and wrote the paper; J.M.B. collected and analysed data.
Competing interests
Neither author reports any competing interests. The authors alone are responsible for the content and writing of the paper.
Funding
This project was partially supported by funding from University of Roehampton.
References
- 1.Hinde RA. 1976. Interactions, relationships and social structure. Man 11, 1–17. ( 10.2307/2800384) [DOI] [Google Scholar]
- 2.Wilson EO. 1975. Sociobology: the new synthesis. Cambridge, MA: Harvard University Press. [Google Scholar]
- 3.Levy DA, Nail PR. 1993. Contagion: a theoretical and empirical review and reconceptualization. Genet. Soc. Gen. Psychol. Monogr. 119, 233–284. [PubMed] [Google Scholar]
- 4.Watson CFI, Caldwell CA. 2010. Neighbor effects in marmosets: social contagion of agonism and affiliation in captive Callithrix jacchus. Am. J. Primatol. 72, 549–558. ( 10.1002/ajp.20805) [DOI] [PubMed] [Google Scholar]
- 5.Christakis NA, Fowler JH. 2013. Social contagion theory: examining dynamic social networks and human behavior. Stat. Med. 32, 556–577. ( 10.1002/sim.5408) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knutson B, Burgdorf J, Panksepp J. 2002. Ultrasonic vocalizations as indices of affective states in rats. Psychol. Bull. 128, 961–977. ( 10.1037//0033-2909.128.6.961) [DOI] [PubMed] [Google Scholar]
- 7.Burman O, Ilyat A, Jones G, Mendl M. 2007. Ultrasonic vocalizations as indicators of welfare for laboratory rats (Rattus norvegicus). Appl. Anim. Behav. Sci. 104, 116–129. ( 10.1016/j.applanim.2006.04.028) [DOI] [Google Scholar]
- 8.Schino G, Troisi A, Perretta G, Monaco V. 1991. Measuring anxiety in nonhuman primates: effect of lorazepam on macaque scratching. Pharmacol. Biochem. Behav. 38, 889–891. ( 10.1016/0091-3057(91)90258-4) [DOI] [PubMed] [Google Scholar]
- 9.Maestripieri D, Schino G, Aureli F, Troisi A. 1992. A modest proposal: displacement activities as an indicator of emotions in primates. Anim. Behav. 44, 967–979. ( 10.1016/S0003-3472(05)80592-5) [DOI] [Google Scholar]
- 10.Schino G, Perretta G, Taglioni AM, Monaco V, Troisi A. 1996. Primate displacement activities as an ethopharmacological model of anxiety. Anxiety 2, 186–191. ( 10.1002/(SICI)1522-7154(1996)2:4%3C186::AID-ANXI5%3E3.0.CO;2-M) [DOI] [PubMed] [Google Scholar]
- 11.Judge PG, Mullen SH. 2005. Quadratic postconflict affiliation among bystanders in a hamadryas baboon group. Anim. Behav. 69, 1345–1355. ( 10.1016/j.anbehav.2004.08.016) [DOI] [Google Scholar]
- 12.Daniel JR, Alves RL. 2015. Postconflict affiliation among bystanders in a captive group of Japanese macaques (Macaca fuscata). Int. J. Primatol. 36, 259–268. ( 10.1007/s10764-015-9822-8) [DOI] [Google Scholar]
- 13.Watson CFI, Buchanan-Smith HM, Caldwell CA. 2014. Call playback artificially generates a temporary cultural style of high affiliation in marmosets. Anim. Behav. 93, 163–171. ( 10.1016/j.anbehav.2014.04.027) [DOI] [Google Scholar]
- 14.Videan EN, Fritz J, Schwandt M, Howell S. 2005. Neighbor effect: evidence of affiliative and agonistic social contagion in captive chimpanzees (Pan troglodytes). Am. J. Primatol. 66, 131–144. ( 10.1002/ajp.20133) [DOI] [PubMed] [Google Scholar]
- 15.Schwing R, Nelson XJ, Wein A, Parsons S. 2017. Positive emotional contagion in a New Zealand parrot. Curr. Biol. 27, R213–R214. ( 10.1016/j.cub.2017.02.020) [DOI] [PubMed] [Google Scholar]
- 16.Seffer D, Schwarting RKW, Wohr M. 2014. Pro-social ultrasonic communication in rats: insights from playback studies. J. Neurosci. Methods 234, 73–81. ( 10.1016/j.jneumeth.2014.01.023) [DOI] [PubMed] [Google Scholar]
- 17.Cherulnik PD, Donley KA, Wiewel TSR, Miller SR. 2001. Charisma is contagious: the effect of leaders' charisma on observers’ affect. J. Appl. Soc. Psychol. 31, 2149–2159. ( 10.1111/j.1559-1816.2001.tb00167.x) [DOI] [Google Scholar]
- 18.Pugh SD. 2001. Service with a smile: emotional contagion in the service encounter. Acad. Manage. J. 44, 1018–1027. ( 10.5465/3069445) [DOI] [Google Scholar]
- 19.van der Gaag C, Minderaa RB, Keysers C.. 2007. Facial expressions: what the mirror neuron system can and cannot tell us. Soc. Neurosci. 2, 179–222. ( 10.1080/17470910701376878) [DOI] [PubMed] [Google Scholar]
- 20.Schnall S, Roper J, Fessler DMT. 2010. Elevation leads to altruistic behavior. Psychol. Sci. 21, 315–320. ( 10.1177/0956797609359882) [DOI] [PubMed] [Google Scholar]
- 21.Nook EC, Ong DC, Morelli SA, Mitchell JP, Zaki J. 2016. Prosocial conformity: prosocial norms generalize across behaviour and empathy. Pers. Soc. Psychol. Bull. 42, 1045–1062. ( 10.1177/0146167216649932) [DOI] [PubMed] [Google Scholar]
- 22.Favreau FR, Goldizen AW, Pays O. 2010. Interactions among social monitoring, anti-predator vigilance and group size in eastern grey kangaroos. Proc. R. Soc. B 277, 2089–2095. ( 10.1098/rspb.2009.2337) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaynor KM, Cords M. 2012. Antipredator and social monitoring functions of vigilance behaviour in blue monkeys. Anim. Behav. 84, 531–537. ( 10.1016/j.anbehav.2012.06.003) [DOI] [Google Scholar]
- 24.Butler SR, Hosinski EC, Lucas JR, Fernández-Juricic E. 2016. Social birds copy each other's lateral scans while monitoring group mates with low-acuity vision. Anim. Behav. 121, 21–31. ( 10.1016/j.anbehav.2016.08.002) [DOI] [Google Scholar]
- 25.Watson CFL. 2011. Social contagion in common marmosets (Callithrix jachus): implications for cognition, culture and welfare. Doctoral dissertation, University of Stirling, Stirling, UK. [Google Scholar]
- 26.Cheney DL, Seyfarth RM. 2009. Stress and coping mechanisms in female primates. Adv. Study Behav. 39, 1–44. ( 10.1016/S0065-3454(09)39001-4) [DOI] [Google Scholar]
- 27.Mooring MS, Blumstein DT, Stoner CJ. 2004. The evolution of parasite-defence grooming in ungulates. Biol. J. Linn. Soc. 81, 17–37. ( 10.1111/j.1095-8312.2004.00273.x) [DOI] [Google Scholar]
- 28.Carter G, Leffer L. 2015. Social grooming in bats: are vampire bats exceptional? PLoS ONE 10, e138430 ( 10.1371/journal.pone.0138430) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stopka P, Macdonald DW. 1999. The market effect in the wood mouse, Apodemus sylvaticus: selling information on reproductive status. Ethology 105, 969–982. ( 10.1046/j.1439-0310.1999.00485.x) [DOI] [Google Scholar]
- 30.Dunbar RIM. 1991. Functional significance of social grooming in primates. Folia Primatol. 57, 121–131. ( 10.1159/000156574) [DOI] [Google Scholar]
- 31.Radford AN. 2012. Post-allogrooming reductions in self-directed behaviour are affected by role and status in the green woodhoopoe. Biol. Lett. 8, 24–27. ( 10.1098/rsbl.2011.0559) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Russell YI, Phelps S. 2013. How do you measure pleasure? A discussion about intrinsic costs and benefits in primate allogrooming. Biol. Phil. 28, 1005–1020. ( 10.1007/s10539-013-9372-4) [DOI] [Google Scholar]
- 33.Boccia ML, Reite M, Laudenslager M. 1989. On the physiology of grooming in a pigtail macaque. Physiol. Behav. 45, 667–670. ( 10.1016/0031-9384(89)90089-9) [DOI] [PubMed] [Google Scholar]
- 34.Aureli F, Preston SD, de Waal FB.. 1999. Heart rate responses to social interactions in free-moving rhesus macaques (Macaca mulatta): a pilot study. J. Comp. Psychol. 113, 59–65. ( 10.1037/0735-7036.113.1.59) [DOI] [PubMed] [Google Scholar]
- 35.Feh C, de Mazierès J.. 1993. Grooming at a preferred site reduces heart rate in horses. Anim. Behav. 46, 1191–1194. ( 10.1006/anbe.1993.1309) [DOI] [Google Scholar]
- 36.Keverne EB, Martensz ND, Tuite B. 1989. Beta-endorphin concentrations in cerebrospinal fluid of monkeys are influenced by grooming relationships. Psychoneuroendocrinology 14, 155–161. ( 10.1016/0306-4530(89)90065-6) [DOI] [PubMed] [Google Scholar]
- 37.Schino G, Scucchi S, Maestripieri D, Turillazzi PG. 1988. Allogrooming as a tension-reduction mechanism: a behavioral approach. Am. J. Primatol. 16, 43–50. ( 10.1002/ajp.1350160106) [DOI] [PubMed] [Google Scholar]
- 38.Aureli F, Yates K. 2010. Distress prevention by grooming others in crested black macaques. Biol. Lett. 6, 27–29. ( 10.1098/rsbl.2009.0513) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shutt K, MacLarnon A, Heistermann M, Semple S. 2007. Grooming in Barbary macaques: better to give than to receive? Biol. Lett. 3, 231–233. ( 10.1098/rsbl.2007.0052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crockford C, Wittig RM, Langergraber K, Ziegler TE, Zuberbuhler K, Deschner T. 2013. Urinary oxytocin and social bonding in related and unrelated wild chimpanzees. Proc. R. Soc. B 280, 20122765 ( 10.1098/rspb.2012.2765) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Waal FBM, Yoshihara D.. 1983. Reconciliation and redirected affection in rhesus monkeys. Behaviour 85, 224–241. ( 10.1163/156853983X00237) [DOI] [Google Scholar]
- 42.Newton-Fisher NE. 2012. Animal Behaviour Pro: v. 1.2. See https://itunes.apple.com/gb/app/animal-behaviour-pro/id579588319?mt=8.
- 43.Carder G, Semple S. 2008. Visitor effects on anxiety in two captive groups of western lowland gorillas. Appl. Anim. Behav. Sci. 115, 211–220. ( 10.1016/j.applanim.2008.06.001) [DOI] [Google Scholar]
- 44.Hohmann G, Mundry R, Deschner T. 2009. The relationship between socio-sexual behavior and salivary cortisol in bonobos: tests of the tension regulation hypothesis. Am. J. Primatol. 71, 223–232. ( 10.1002/ajp.20640) [DOI] [PubMed] [Google Scholar]
- 45.O'Neill WM, Hanks GW, Simpson P, Fallon MT, Jenkins E, Wesnes K. 2000. The cognitive and psychomotor effects of morphine in healthy subjects: a randomized controlled trial of repeated (four) oral doses of dextropropoxyphene, morphine, lorazepam and placebo. Pain 85, 209–215. ( 10.1016/S0304-3959(99)00274-2) [DOI] [PubMed] [Google Scholar]
- 46.de Waal FBM, Preston SD.. 2017. Mammalian empathy: behavioural manifestations and neural basis. Nat. Rev. Neurosci. 18, 498–509. ( 10.1038/nrn.2017.72) [DOI] [PubMed] [Google Scholar]
- 47.Briefer EF. 2018. Vocal contagion of emotions in non-human animals. Proc. R. Soc. B 285, 20172783 ( 10.1098/rspb.2017.2783) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mendl M, Burman OHP, Paul ES. 2010. An integrative and functional framework for the study of animal emotion and mood. Proc. R. Soc. B 277, 2895–2904. ( 10.1098/rspb.2010.0303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kranjec J, Beguš S, Geršak G, Drnovšek J. 2014. Non-contact heart rate and heart rate variability measurements: a review. Biomed. Signal Process. Control 13, 102–112. ( 10.1016/j.bspc.2014.03.004) [DOI] [Google Scholar]
- 50.Wittig RM, Crockford C, Weltring A, Langergraber KE, Deschner T, Zuberbühler K. 2016. Social support reduces stress hormone levels in wild chimpanzees across stressful events and everyday affiliations. Nat. Commun. 7, 13361 ( 10.1038/ncomms13361) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coussi-Korbel S, Fragaszy DM. 1995. On the relation between social dynamics and social learning. Anim. Behav. 50, 1441–1453. ( 10.1016/0003-3472(95)80001-8) [DOI] [Google Scholar]
- 52.Barsade SG. 2002. The ripple effect: emotional contagion and its influence on group behavior. Adm. Sci. Quart. 47, 644–675. ( 10.2307/3094912) [DOI] [Google Scholar]
- 53.Gomes CM, Mundry R, Boesch C. 2009. Long-term reciprocation of grooming in wild West African chimpanzees. Proc. R. Soc. B 276, 699–706. ( 10.1098/rspb.2008.1324) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ventura R, Majolo B, Koyama NF, Hardie S, Schino G. 2006. Reciprocation and interchange in wild Japanese macaques: grooming, cofeeding, and agonistic support. Am. J. Primatol. 68, 1138–1149. ( 10.1002/ajp.20314) [DOI] [PubMed] [Google Scholar]
- 55.Carne C, Wiper S, Semple S. 2011. Reciprocation and interchange of grooming, agonistic support, feeding tolerance, and aggression in semi-free-ranging Barbary macaques. Am. J. Primatol. 73, 1127–1133. ( 10.1002/ajp.20979) [DOI] [PubMed] [Google Scholar]
- 56.Henzi SP, Barrett L. 2002. Infants as a commodity in a baboon market. Anim. Behav. 63, 915–921. ( 10.1006/anbe.2001.1986) [DOI] [Google Scholar]
- 57.Gumert MD. 2007. Payment for sex in a macaque mating market. Anim. Behav. 74, 1655–1667. ( 10.1016/j.anbehav.2007.03.009) [DOI] [Google Scholar]
- 58.Seyfarth RM, Cheney DL. 1984. Grooming, alliances and reciprocal altruism in vervet monkeys. Nature 308, 541–543. ( 10.1038/308541a0) [DOI] [PubMed] [Google Scholar]
- 59.Hirsch BT, Stanton MA, Maldonado JE. 2012. Kinship shapes affiliative social networks but not aggression in ring-tailed coatis. PLoS ONE 7, e37301 ( 10.1371/journal.pone.0037301) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kern JM, Radford AN. 2018. Experimental evidence for delayed contingent cooperation among wild dwarf mongooses. Proc. Natl Acad. Sci. USA 115, 6255–6260. ( 10.1073/pnas.1801000115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Massen JMM, Szipl G, Spreafico M, Bugnyar T. 2014. Ravens intervene in others' bonding attempts. Curr. Biol. 24, 2733–2736. ( 10.1016/j.cub.2014.09.073) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Krueger K, Flauger B, Farmer K, Hemelrijk C. 2014. Movement initiation in groups of feral horses. Behav. Proc. 103, 91–101. ( 10.1016/j.beproc.2013.10.007) [DOI] [PubMed] [Google Scholar]
- 63.Mielke A, Samuni L, Preis A, Gogarten JF, Crockford C, Wittig RM. 2017. Bystanders intervene to impede grooming in Western chimpanzees and sooty mangabeys. R. Soc. open sci. 4, 171296 ( 10.1098/rsos.171296) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mielke A, Preis A, Samuni L, Gogarten JF, Wittig RM, Crockford C. 2018. Flexible decision-making in grooming partner choice in sooty mangabeys and chimpanzees. R. Soc. open sci. 5, 172143 ( 10.1098/rsos.172143) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kaburu SSK, Newton-Fisher NE. 2016. Bystanders, parcelling, and an absence of trust in the grooming interactions of wild male chimpanzees. Sci. Rep. 6, 20634 ( 10.1038/srep20634) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Newton-Fisher NE, Kaburu SSK. 2017. Grooming decisions under structural despotism: the impact of social rank and bystanders among wild male chimpanzees. Anim. Behav. 128, 153–164. ( 10.1016/j.anbehav.2017.04.012) [DOI] [Google Scholar]
- 67.Rueckert L, Naybar N. 2008. Gender differences in empathy: the role of the right hemisphere. Brain Cogn. 67, 162–167. ( 10.1016/j.bandc.2008.01.002) [DOI] [PubMed] [Google Scholar]
- 68.Clay Z, de Waal FBM. 2013. Bonobos respond to distress in others: consolation across the age spectrum. PLoS ONE 8, e55206 ( 10.1371/journal.pone.0055206) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hurlemann R, Patin A, Onur OA, Cohen MX, Baumgartner T, Metzler S, Kendrick KM. 2010. Oxytocin enhances amygdala-dependent, socially reinforced learning and emotional empathy in humans. J. Neurosci. 30, 4999–5007. ( 10.1523/JNEUROSCI.5538-09.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Treated data are available in the electronic supplementary material; all raw data files are deposited at https://figshare.com/articles/Berthier_and_Semple_-_raw_data_files/7029269.