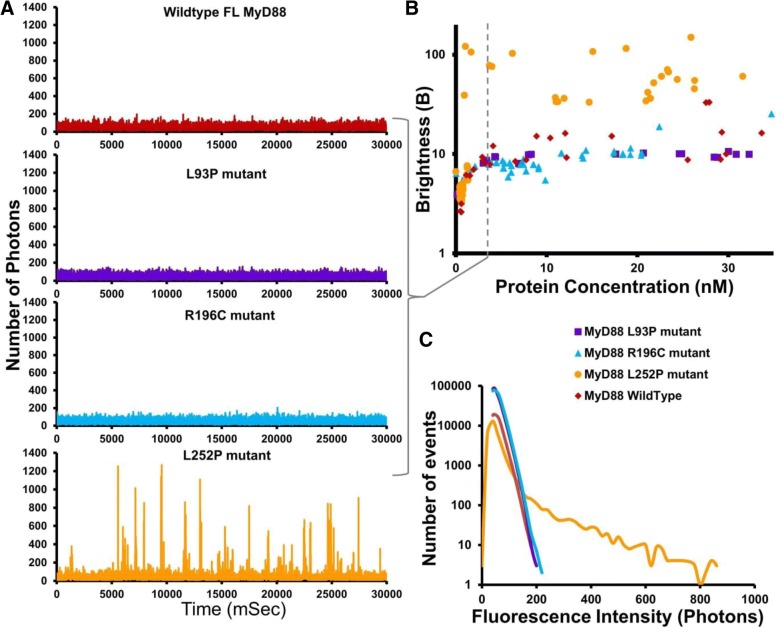
Fig. 5.
Mutations in same domain lead to contrasting disease phenotypes; cancer-causing L252P mutation lowers threshold for MyD88 oligomerisation. a Fluorescence time-traces obtained at 3 nM protein concentration of the disease-associated point mutants in the full-length MyD88 protein, as well as full-length wild-type MyD88, demonstrating the stability of the L252P point mutant. b The B parameter (brightness) correlates with the number of oligomers detected in typical time-traces as a function of protein concentration (nM). c Fluorescence intensity histogram demonstrating the stable L252P oligomer still forming at 3 nM, in comparison to the other constructs. Fluorescence time-traces in a are representative traces obtained at 3 nM protein concentrations, c is the representative fluorescence intensity histogram. Values in b are from approx. 60 dilution experiments with the various protein concentrations and corresponding brightness values obtained plotted. Fluorescence intensity values at 3 nM are statistically significant with P < 0.0001 between L252P mutant values and the other mutants