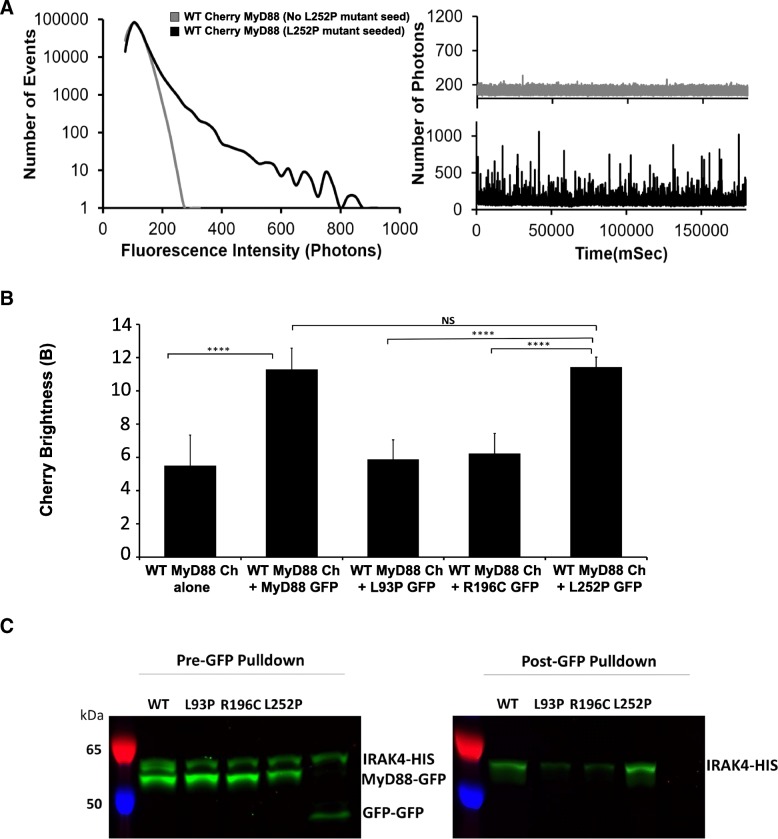
Fig. 7.
Gain of function point mutation, L252P, recruits both wildtype MyD88 and downstream IRAK4. a Example fluorescence intensity histogram showing the impact of the L252P point mutation on WT FL MyD88. Corresponding fluorescence time-traces obtained for full-length mCherry MyD88 protein at subcritical concentration (5 nM) and then with the addition of full-length L252P mutant GFP seed. The diffusion of the Cherry MyD88 protein equates to the same number of fluorophores moving through the confocal volume, creating a burst of fluorescence in the time-trace being directly proportional to the size of the oligomer. b Brightness histogram of the mCherry-tagged wild-type MyD88 (expressed at subcritical concentration) co-expressed with disease-associated mutants (simulating heterozygous expression in patients), as well as L93P, R196C or L252P mutant proteins co-expressed with themselves (i.e. homozygous protein expression) and wild-type MyD88 alone as a control. mCherry brightness from WT MyD88 measured in three independent experiments. NS > 0.9999, ***P = 0.0001, ****P < 0.0001. c GFP pulldown of GFP-tagged MyD88 WT, L93P, R196C or L252P mutant proteins co-expressed with IRAK4-HIS tagged. IRAK4-HIS tagged 58 kDa, MyD88-GFP tagged 53.2 kDa, GFP dimer control 40 kDa. Pre-pulldown and post-pulldown shown for MyD88 WT, L93P, R196C, L252P mutant and GFP dimer control. GFP not visible in post-pulldown due to boiling step. HIS-tagged IRAK4 labelled with bodypi. Example gel from three independent experimental repeats