Abstract
Menstrual blood is a unique body fluid that contains mesenchymal stem cells (MSCs). These cells have attracted a great deal of attention due to their exceptional advantages including easy access and frequently accessible sample source and no need for complex ethical and surgical interventions, as compared to other tissues. Menstrual blood-derived MSCs possess all the major stem cell properties and even have a greater proliferation and differentiation potential as compared to bone marrow-derived MSCs, making them a perspective tool in a further clinical practice. Although the potential of menstrual blood stem cells to differentiate into a large variety of tissue cells has been studied in many studies, their chondrogenic properties have not been extensively explored and investigated. Articular cartilage is susceptible to traumas and degenerative diseases, such as osteoarthritis, and has poor self-regeneration capacity and therefore requires more effective therapeutic technique. MSCs seem promising candidates for cartilage regeneration; however, no clinically effective stem cell-based repair method has yet emerged. This chapter focuses on studies in the field of menstrual blood-derived MSCs and their chondrogenic differentiation potential and suitability for application in cartilage regeneration. Although a very limited number of studies have been made in this field thus far, these cells might emerge as an efficient and easily accessible source of multipotent cells for cartilage engineering and cell-based chondroprotective therapy.
1. Introduction
Mesenchymal stem cells (MSCs) with their multipotent differentiation capability attract a lot of attention from researchers, developing possible ways of employing these cells in clinical practice. MSCs have been isolated and studied from different sources, including bone marrow, adipose tissue, synovial membrane, umbilical cord, and dental pulp [1]. The bone marrow is the primary tissue where MSCs were firstly isolated in 1957 and is considered to be a classical MSC source, which is often used as a control for other source MSCs [2].
In 2007, Meng with colleagues isolated a MSC population from menstrual blood (MenSC) [3]. MSC properties, including multiple differentiation, have been confirmed for these cells, while their differentiation capability and multipotency were even greater than bone marrow-derived MSCs (BMMSCs), suggesting that MenSCs are potent candidates for clinical applications. Furthermore, MenSCs are much easier to access compared to BMMSCs as their collection does not require complicated ethical procedures or any invasive surgical interventions, thus providing an option of repeated sample collection in the same donor. These advantages suggest MenSCs as an attractive tool for regenerative medicine.
Articular cartilage is an avascular load-bearing connective tissue with unique mechanical properties. However, the cartilage is a poor self-regenerating tissue and is highly susceptible to trauma or degenerative diseases such as osteoarthritis (OA), which is characterised by varying degrees of physical and functional limitation and reduced quality of life, with a major impact on the quality of life of the ageing population in European countries [4]. The cartilage is populated exclusively by chondrocytes; however, its regenerative capacities are limited due to a complicated extracellular matrix (ECM) structure and difficulties associated with repopulating the cells within the tissue after trauma and inflammation [5]. Currently, there is no efficient therapeutic approach for cartilage lesions, and cell-based therapies such as multipotent MSCs from different sources seem promising candidates for cartilage tissue engineering and stimulation of cartilage regeneration [6, 7]. Although the majority of therapeutic techniques using MSCs produce poor outcomes with limited success rates, these cells remain a key focus on studies aimed at differentiating them into a robust chondrogenic lineage and establishing novel protocols for clinical studies.
The main goal of stimulating a qualitative chondrogenic response in cells is to select an appropriate protocol to induce cell cascades responsible for chondrogenesis. One of the major components of all chondrogenic differentiation media is the growth factor transforming growth factor β (TGF-β), which is crucial for in vivo and in vitro chondrogenesis; however, other factors which also play an important role in this process are not always involved in stimulating MSCs to differentiate. In fact, different tissue MSCs might require novel protocols with different biologically active factors, optimized for a correct tissue source MSCs, which may reveal stronger effects in cell chondrogenic response.
Although MenSCs are known to have a great potential to differentiate into various tissue cells, their chondrogenic differentiation potential has not been extensively investigated so far. In this review, we aim to gather all up-to-date knowledge considering MenSC potential to differentiate into chondrogenic lineage. Currently, BMMSCs have been considered as the most potential candidates for cartilage regeneration techniques; however, these cells deploy a number of disadvantages in their usage, including invasive and painful sample collection, shortage of biological material, and small number of cells in it, whereas, those issues are not relevant to MenSCs.
2. MenSC Characteristics
The female reproductive system is a complicated combination of biological components where the uterine endometrium plays an exclusive role. This fast-regenerating tissue has been considered as a source for easy-accessible stem cells decades ago [8]. It is known that the endometrium undergoes over 400 cycles of regeneration and menstruation during a woman's reproductive life cycle, allowing for pregnancy, and can be even continued to regenerate after menopause using estrogen therapy [9]. It was repeatedly confirmed that the endometrium is rich with epithelial progenitor cells as well as MSCs [10–12]. Moreover, endometrium MSCs (EnSCs) have been shown to regenerate into all three different layers—endoderm, mesoderm, and ectoderm—and maintain similar properties to BMMSCs [11, 12]. EnSCs can be isolated directly from the endometrium using hysterectomy or endometrial biopsy; however, these procedures are invasive and require surgical intervention. Another way of collecting EnSCs is their isolation from menstrual blood, which is being naturally discarded from organism each month as waste and requires minimal ethical issues.
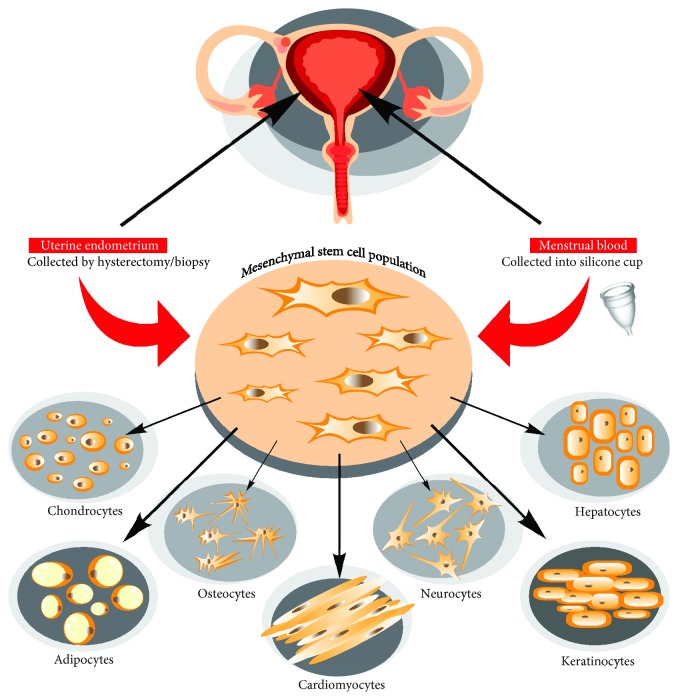
Menstrual blood-derived EnSCs (MenSCs) were firstly observed by Meng and his team in 2007. From that time, this source of collecting MSCs has attracted huge scientific interest, leading to a number of different research avenues and possible applications of MenSCs in clinical practice. It has been shown that MenSCs possess such typical MSC qualities as self-renewal, high proliferative potential, and a multipotent differentiation ability into chondrogenic, adipogenic, and osteogenic lineages in vitro [13], (see Figure 1).
Figure 1.
Mesenchymal stem cell isolation from uterine endometrium and menstrual blood and their differentiation potential.
3. Differences between BMMSC and MenSC Phenotypes and Differentiation Potentials
BMMSCs are a classical MSC population, which is often employed as a reference control for evaluation of phenotype and functional peculiarities of other sources of MSCs. Although MenSCs share a lot of similar typical properties with BMMSCs, MenSCs seem to have some advantageous characteristics. For instance, recent studies have shown that MenSCs are even able to differentiate into cardiomyocytes with the functions of beating spontaneously after induction resulting in the decreased myocardial infarction area in a rat model [14, 15]. Furthermore, it has been shown that MenSCs are capable to differentiate into neural and epidermal-like cells [16–19] and even functional hepatocytes [20], which suggest superior spectrum of their differentiation potential, as compared to BMMSCs (Figure 1). In addition to the whole range of MSC surface markers, including CD73, CD90, and CD105, MenSCs also express some pluripotency markers, such as OCT-4, SSEA-4 [17, 21], highly upregulated levels of CD49a [22] but lack of STRO1 expression [23, 24], which further distinguishes them from BMMSCs. Furthermore, it has been shown that the proliferation capability of MenSCs is much higher than that of BMMSCs [3, 23, 24]. Colony forming unit (CFU) rate and proangiogenic capacity in vitro have been also established as much higher in MenSCs as compared to BMMSCs [22]. Lower tumorigenicity has been reported for MenSCs, as compared to BMMSCs, which implies safety of MenSC-based therapies [20, 25]. These findings support MenSCs as a unique and promising cell population; however, the beneficial clinical efficacy of those cells in comparison to BMMSCs remains to be investigated.
4. Articular Cartilage and Its Regenerative Disability—Stem Cells Might Be an Answer
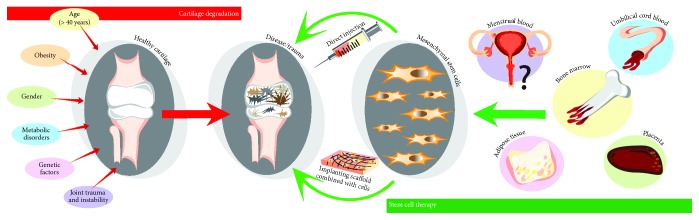
Articular cartilage, due to its low capacity for self-repair, is highly susceptible to trauma or degenerative low-grade inflammatory diseases such as OA, leading to disability and the loss of quality of life in a considerable part of population worldwide. In 2014, there has been registered more than 237 million (3.3%) of the world's population that are suffering from OA [26]. The prevalence of OA increases with age: 13.9% of adults at age 25 years, while 33.6% of adults at age 65 and older have OA, where more than a half of them are women. This gender difference is important and relevant to the topic of this review. The major factors that increase the risk of OA are age, obesity, gender, joint disease, or abnormalities with its functions, metabolic disorders, and genetic factors [27] but gender is especially important after menopause.
Age is the primary factor for OA, as it usually forms in the 40s onwards. Obesity creates a harmful load on joints and has a negative influence on cartilage, increasing the chance of developing OA and even getting it worse with time [28]. Moreover, according to statistics, OA is most common and severe in women and any kind of surgical operation on a joint can lead to OA [29]. Furthermore, metabolic disorders have also been considered as one of the causes for OA. Altered metabolic pathways and mediators in OA cartilage have been even highlighted as potential therapeutic targets [30]. Equally, alterations in the ion channels that enable Ca2+ transport across the plasma membrane seem to be critical for the development of cartilage degeneration in OA [5, 31]. Although all of these factors have been extensively studied, the knowledge has not been translated to therapies—there are still no efficient cell-based therapeutic approaches for cartilage lesions. Cell-based therapies such as multipotent MSCs seem promising candidates for cartilage engineering and regeneration [32]. Tissue engineers have constructed different ways of a possible cartilage treatment with MSCs, including direct injection into cartilage, mixing them with hydrogels, or seeding on scaffolds [33] (see Figure 2). BMMSCs have been identified as the most popular choice for cartilage tissue regeneration techniques due to their plasticity and close location to the cartilage. Furthermore, the placenta, umbilical cord blood, and adipose tissues were also used as MSC sources in cartilage tissue engineering [34, 35].
Figure 2.
Major OA risk factor strategies for promoting cell-based cartilage repair.
However, the majority of cartilage engineering or repair techniques using MSCs have failed so far due to a number of complicating factors, such as development of hypertrophy [36]. Hypertrophy is often acquired in MSCs during chondrogenic induction, leading to a possible further differentiation to endochondral bone formation. It is marked by sudden increase in cell volume (more than 10-fold) and structural remodelling of ECM, forming calcification and mineralization of ECM. Cells begin to synthesize collagen type X, produce destructive metalloproteinases. Therefore, hypertrophy affects not only chondrocyte homeostasis but also cartilage structure [37]. Furthermore, there are other MSC application restrictions, as isolating them from a large number of donors, small amount of available cells, and decrease in their proliferation/differentiation rate with age [9].
Menstrual blood is a unique easily accessible source of stem cells, which eliminates the majority of BMMSCs and other tissue MSC restrictions and can be used to treat different diseases, where OA might not be an exception. Although MenSCs were not applied in cartilage regeneration techniques yet, their candidature in these procedures is high. For instance, to the best of our knowledge, there is no data concerning potential formation of fibrocartilage (collagen type I) or hypertrophy (collagen type X, VEGF, MMP-13) during chondrogenesis in MenSCs, which might appear an additional advantage for their application for cartilage repair.
Noteworthily, it is logical to assume that the ability to collect menstrual blood for autologous treatment with MenSCs is progressively reduced in elderly women which could appear a limitation for their therapeutic applications in OA. On the other hand, if these cells could be collected and cryopreserved in advance, there will always be an opportunity to use them later in the donor's lifetime. Moreover, MenSCs are derived from shedding endometrium, suggesting that if the endometrium can maintain its regenerative capabilities even after menopause, this may prolong and sustain stem cell collection, allowing application of autologous biological material in future clinical therapies even for elderly women [25].
5. Chondrogenesis and Impact of MSCs
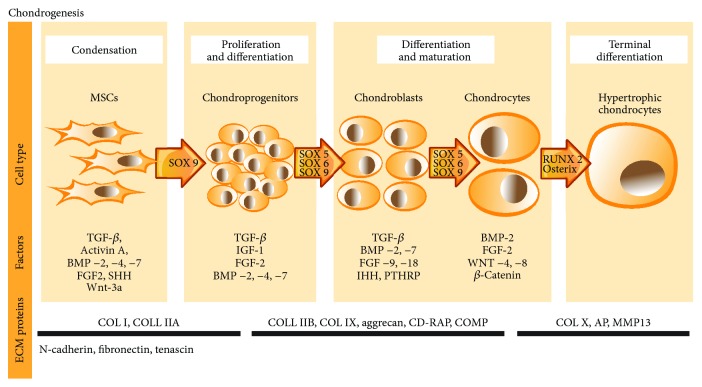
Molecular mechanisms that control chondrogenic differentiation in MSCs have been the major focus of research and important puzzle to solve for exploiting biochemical pathways to induce cartilage regeneration. In vivo chondrogenesis is initiated by several growth factors, such as tumor growth factors-β (TGF-βs), Activin A, bone morphogenetic proteins- (BMP-) 2, BMP-4, BMP-7, and fibroblast growth factors (FGFs) [38]. TGF-β is critical for chondrogenesis as it is considered to be a crucial stimulator for chondrogenic differentiation both in vitro and in vivo [32]. TGF-βs (mainly TGF-β1 and TGF-β3) stimulate chondrogenesis through SMAD3 protein, which further stimulates transcriptional activity of Sox9 leading to activation of cartilage-specific protein genes, as type II and type IX collagen, aggrecan, CD-RAP, and cartilage oligomeric protein (COMP) [39]. FGFs have been shown to promote chondrocyte proliferation in vivo. FGF-2, FGF-9, and FGF-18 are the most studied growth factors in chondrogenesis, where FGF-2 upregulates Sox9 and early activation of chondrogenesis and FGF-9/18 maintain chondrocyte phenotype, delaying hypertrophy [36, 40]. Furthermore, FGFs often act in concert with other growth factors such as insulin-like growth factors (IGFs) that are required for a proper chondrogenesis formation, as well as cell proliferation and motility. IGF-1 was found to be equally potent to TGF-β1 in chondroinductive actions of BMMSCs (Longorbardi et al., 2006). Moreover, it enhances cartilage matrix formation, regulates apoptosis, and blocks interleukin-1-induced turnover of proteoglycans in chondrocytes, which makes this factor an important element in chondrogenesis (Chun du oh, 2003). Wingless proteins (Wnts) are important in a variety of cellular activities during chondrogenic differentiation, including proliferation and gene expression, as they induce production of FGFs [41–43]. Sonic hedgehog (SHH) induces MSCs to synthesize BMPs, directing MSC differentiation into chondrogenic lineage [44]. Furthermore, several factors maintain the chondrocyte phenotype in the cartilage, such as parathyroid-related peptide (PTHRP) and Indian hedgehog (IHH) [44].
All of these growth factors play a key role in tissue repair and regeneration, and most importantly—these are crucial factors in all chondrogenesis stages [45, 46] (see Figure 3). Transcription factors also play an essential role in chondrogenesis as they regulate not only the expression of ECM proteins but also the expression of growth factors according to the differentiation stage. Sox9 is one of the earliest markers expressed in the MSCs and is the key transcription factor in chondrocyte maturation [47]. Sox5 and Sox6 maintain chondrocyte phenotype at later stages and directly regulate expression of ECM molecules, such as collagen (IIB, IX, X) and proteoglycans (aggrecans) [48]. RunX2 and osterix negatively affect chondrogenesis, as they induce mineralization of the cartilage matrix [49], by promoting matrix metalloproteinase 13 (MMP13) synthesis [50]. Generally, MMP synthesis in cells is stimulated by proinflammatory cytokines, allowing them to negatively regulate cell processes. In the cartilage, MMPs (mainly MMP-9, MMP-10, MMP-13, and MMP-14) lead chondrocytes to hypertrophy and remodel ECM, forming cartilage degradation [44].
Figure 3.
Stages of chondrogenesis in vivo.
In the meantime, classical chondrogenic differentiation medium consists of a combination of growth factors (predominantly TGF-βs), ITS, high-glucose, dexamethasone, ascorbic acid-phosphate, sodium pyruvate and proline, and in major cases—lacks serum. These factors along with natural stem cells secreting biologically active compounds stimulate their differentiation towards chondrogenic lineage. For this reason, before applying stem cells in tissue regeneration techniques, it is useful and important to evaluate their secretome profile.
MSCs are beneficial for OA repair techniques due to their anti-inflammatory and chondroprotective properties. They are known to secrete a broad range of various paracrine factors and bioactive molecules that can modulate metabolism of extracellular matrix in OA cartilage [7]. Cytokines are major factors that regulate cell differentiation capabilities. BMMSC secretome was characterised in many studies. It was found that BMMSCs secrete a wide range of different cytokines/growth factors, including interleukins: IL-6, IL-7, IL-8, IL-11, IL-12, IL-14, IL-15, leukemia inhibitory factor (LIF), granulocyte colony-stimulating factor (G-CSF), granulocyte macrophage colony-stimulating factor (GM-CSF), macrophage colony-stimulating factor (M-SCF), Flt-3 ligand (FL), and stem cell factor (SCF) [51].
6. Growth Factors Secreted by MenSCs and Their Potential Impact on Chondrogenic Differentiation
As for MenSCs, their secretome is less studied; however, several studies already published their results according to MenSC cytokine and growth factor secretion, cultivating them in monolayer (see Table 1).
Table 1.
The analysis of MenSC secretome in published studies∗.
| Analyzed cytokines/growth factors | MenSCs positive for | Conclusion | Reference |
|---|---|---|---|
| MMP-3, MMP-10, GMCSF, PDGF-BB, ANG-2, VEGF, HGF, EGF | MMP-3, MMP-10, GM-CSF, PDGF-BB, ANG-2, VEGF, HGF, EGF | MenSCs share some properties of mesenchymal stem cells based on phenotype but functionally produce factors that are unique. | [3] |
| VEGF, BDNF, GDNF, NT-3 | VEGF, BDNF, NT-3 | Oxygen glucose deprivation (OGD) conditions showed upregulation of VEGF, BDNF, and NT-3 in MenSCs, comparing to normal condition cultivation. | [8] |
| IL-10, IFN-γ, MCP-1, IDO1, COX-2, FOXP3 | IDO1, COX-2, FOXP, IFN-γ, IL-10, MCP-1 | MenSCs from patients with endometriosis express higher amounts of IDO1, IFN-γ, MCP-1, and IL-10. | [52] |
| Activin A, IL-6, Cox2, IDO, PDL-1 | IL-6, Cox2, Activin A, IDO, PDL-1 | MenSCs are less responsive to cytokine activation and express less immunosuppressive molecules compared to BMMSCs. | [22] |
| VEGF, HGF, IGF-1 | VEGF, HGF, IGF-1 | MenSCs make a significant stem cell population, producing cytokines, crucial for tissue repair and regeneration. | [53] |
| VEGF, FGF, KGF, HGF | VEGF, FGF-2, KGF, HGF | MenSCs secrete higher concentration of HGF than from dental pulp—MSCs at the sixth and tenth passage and had the lowest concentration in FGF (from P2 to P10). | [25] |
| MCP-1, IL-6, HGF, GRO, IL-8, OPG | MCP-1, IL-6, HGF, GRO, IL-8, OPG | MenSCs have a potential for reducing liver fibrosis in mice. | [54] |
∗Abbreviations: BDNF: brain-derived neurotrophic factor; Cox: cyclooxygenase; EGF: epidermal growth factor; FGF: fibroblast growth factor; FOX: forkhead transcription factor; GDNF: glial cell line-derived neurotrophic factor; GMCSF: granulocyte macrophage colony-stimulating factor; GRO: growth-related oncogene; HGF: hepatocyte growth factor; IDO: indoleamine 2,3 dioxygenase; IFN: interferon; IGF: insulin-like growth factor; IL: interleukin; KGF: keratinocyte growth factor; MCP: monocyte chemoattractant protein; MMP: metalloprotease; NT: neurotrophin; ANG: angiogenic factor; OPG: osteoprotegerin; PDGF: platelet-derived growth factor; PDL: programmed cell death-ligand; VEGF: vascular endothelial growth factor.
It has been observed that among basal proliferative, angiogenetic, and chemo-attractive proteins, such as VEGF, PDGF, HGF, and ANG-2, MenSCs secrete biologically active molecules IGF-1 and FGF-2, which are involved in different stages of chondrogenesis (Figure 3) [25, 53], as described earlier. Furthermore, MenSCs express Activin A, which is a member of the TGF-β superfamily. Several studies suggest that Activin A plays a pivotal role in the early stages of MSC chondrogenesis [55, 56]. Activin A induces the expression of Oct4, Nanog, Nodal, Wnt3, and FGF8 and is necessary for the maintenance of self-renewal and pluripotency of MSC [55]. Enhanced production of Activin A was demonstrated in OA cartilage, associated with the suppression of aggrecanase-mediated cleavage of aggrecan in human articular cartilage [57], suggesting a chondroprotective role of Activin A during destructive OA process. Chimeric ligands of Activin A and BMP-2 have been used to induce chondrogenic differentiation in adipose tissue-derived MSCs (ASCs) resulting in Peran et al. [56]. They demonstrated increased expression of collagen type 2, Sox9, and aggrecan in ASCs (toluidine blue and Masson's trichrome staining), which was also confirmed by RT-PCR in response to Activin A/BMP-2 chimeras [56]. Besides, Activin A is involved in regulation of women menstrual cycle [58] suggesting that it may appear pivotal for modulation of MenSC differentiation potential. Our preliminary data also confirmed chondrogenic differentiation capacity of MenSCs and its modulation by Activin A (unpublished data).
Conversely, MenSCs secrete immunomodulating factors as IL-6, IL-8, IL-10, IFN-γ, GRO, OPG, HGF, and MCP-1, which take place in an inflammatory process. These factors were studied according to immunosuppressive properties of MenSCs and BMMSCs, which were analyzed in collagen-induced arthritis model in mice and their secreted factors, activated with/without IFN-c and IL-1b. The study concluded that MenSCs are less responsive to cytokine activation and express less immunosuppressive molecules compared to BMMSCs [22], which is not an advantageous fact if considering their applicability in cartilage regeneration. Moreover, MenSCs are shown to express matrix metalloproteases (MMP-3, MMP-10) [3]. The secretion of these factors is considered to negatively affect chondrogenesis in these cells, as they promote chondrocyte hypertrophy, as described earlier.
Nevertheless, these are only few studies made in the field of MenSC secretome. This niche requires more studies to truly understand the nature of these cells and their secreting factors, which can possibly approve or disprove already published results.
7. MenSC Chondrogenic Differentiation Capability for Tissue Engineering Approaches
MenSCs are known to have a great potential to differentiate into various tissue cells; however, their chondrogenic differentiation potential has not been extensively investigated so far. The primary study which analyzed ESC chondrogenic differentiation potential in time was made in 2007 by Wolff and colleagues [59]. They reported that ESC pellets cultured in chondrogenic media secreted proteoglycan as the extracellular matrix was stained with Alcian blue, while control pellets were cultivated in chondrogenic media without growth factors and in DMEM did not. They concluded that endometrial stem cells are capable to differentiate into chondrogenic lineage and that there is a coherence between the staining intensity and differentiation time; for instance, as longer pellets were differentiated, the more proteoglycan were accumulated. In Table 2, we have summarized data from all published studies in the field of MenSC chondrogenic differentiation, including the exact methodologies used by the authors including growth factors and differentiation duration.
Table 2.
Evidence of MenSC chondrogenic differentiation.
| Method | Visualization with | Growth factors/other components used | Duration | Results | Reference |
|---|---|---|---|---|---|
| 2D | Alcian blue | TGF-β3 | 14–20 days | Alcian blue positive | [60] |
| Alcian blue, RT-PCR for collagen type II and Sox9 | TGF-β3, BMP-6, fibronectin-coated | 21 days | Alcian blue positive, collagen type 9 and Sox9 positive, collagen type II negative | [24] | |
| IHC collagen type II antibody | TGF-β3, BMP-6 | 21 days | Collagen type II positive | [52] | |
| Alcian blue | TGF-β3 | 14 days | Alcian blue positive | [20] | |
| Alcian blue | TGF-β3 | 21 days | Alcian blue positive | [53] | |
|
| |||||
| 3D | Alcian blue, IHC with collagen type II antibody | TGF-β3, IGF-1, nanofibrous scaffolds | 4 weeks | Alcian blue and collagen type II positive | [11] |
| Alcian blue, IHC with collagen type II and type I antibodies | TGF-β3, nanofibrous scaffolds | 3 weeks | Alcian blue and collagen type II positive, collagen type I negative | [23] | |
| Alcian blue | TGF-β, BMP-6 | 21 days | Alcian blue positive | [61] | |
| IHC with collagen type II antibodies | TGF-β3, IGF-1 | 4 weeks | Collagen type II positive | [62] | |
| Safranin O, collagen type II RNA gene analysis | TGF-β3 | 21 days | Safranin O and collagen type II gene positive | [22] | |
According to these studies, MenSCs revealed different results in chondrogenic response. Several published studies suggest that MenSCs could be a suitable candidate for cartilage tissue engineering and may have direct effects on cartilage tissue repair, as determined by sulfated glycosaminoglycans and express collagen type II [20, 21]. Other authors suggest that MenSCs have low chondrogenic differentiation potential and are not a suitable stem cell population for cartilage regeneration. For instance, in 2015, there was a study published where authors compared gene expression between MenSCs and umbilical cord MSCs (UCMSCs) from the same donor and between MenSCs and BMMSCs from the same donor. They screened 768 genes in MenSCs, UCMSCs, and BMMSCs. Furthermore, they report that important osteogenic and chondrogenic genes POSTN and OSTM1 were largely downregulated in MenSCs compared with UCMSCs and BMMSCs, which also confirmed the inferior osteogenic and chondrogenic differentiation potentials of MenSCs [18]. However, these authors did not induce their cells to differentiate, which is uncertain due to changes in chondrogenic genes during differentiation process. POSTN gene codes periostin, which was shown to promote osteogenic differentiation [63], inducing ECM mineralization but not chondrogenic differentiation [64]. Furthermore, the expression of these genes in cells is upregulated during differentiation process, so it is unclear what the true expression of these genes is during chondrogenic induction.
On the other hand, the majority of authors claim that differentiated MenSCs (ESCs) showed strong immunoreactivity to a monoclonal antibody against Collagen type 2 and accumulation of proteoglycan that were revealed by Alcian blue staining [24, 59, 60, 62], which are believed to be considerable confirmation of chondrogenesis. Moreover, the comparison of differentiated MenSCs and BMMSCs showed a similar pattern of proteoglycan accumulation [24]. However, the expression of Collagen 2A1 mRNA was particularly observable in differentiated BMMSCs, although not in MenSCs [24], which can be related to inappropriate growth factor induction, which the authors used—TGF-β3, BMP-6. Nevertheless, during MenSC differentiation, they detected a significant increase in the expression level of Collagen 9A1 and the transcription factor SOX9, suggesting that these cells positively respond to chondrogenic induction. Moreover, considering different growth factor influence on chondrogenic differentiation, it is important to note that there are studies suggesting that TGF-β3 does not always induce chondrogenesis in such cells as ADSC and BMMSCs, where BMP-2 was shown to act as a major chondrogenic differentiation inducer in ADSC [65, 66], and the combination of TGF-β1, GDF-5, and BMP-2 stimulated robust chondrogenic response in BMMSCs [67]. These observations may lead to the development of new strategies for novel chondrogenic differentiation protocols for MenSCs, which will include additional factors that these cells may require. For instance, Activin A is known to be crucial in the early stages of chondrogenesis, as described earlier; however, classical chondrogenic differentiation medium does not contain it. Additional growth factors might be useful in MenSC differentiation capability.
8. Conclusions
Menstrual blood is a unique body fluid that contains multipotent cells with typical characteristics of MSCs, while with a greater proliferative and differentiation capability than classical bone marrow-derived MSCs (BMMSCs). These advantages, as well as the ease of access of MenSCs due to possibility of repeated noninvasive menstrual blood sample collection even from the same donor, make MenSCs a promising cellular source for regenerative medicine applications.
Although these cells have many more benefits comparing to other tissue MSCs, some of the research niches still need further investigation to fully identify the applicability of MenSCs for basic research and clinical applications. One of these niches is their chondrogenic differentiation. As articular cartilage has difficulties in self-regeneration and is susceptible to OA, especially in women, MenSCs could serve as a perfect stem cell therapy tool for cartilage regeneration. However, the chondrogenic differentiation potential of MenSCs remains controversial. One concept and claim is that these cells have a strong potential to differentiate, as they efficiently produce proteoglycans and collagen type II [20, 22–24, 62] and may have direct effects on cartilage tissue repair. Another concept is that MenSCs have a weak chondrogenic response [18]. Induction of relevant differentiation cascades in those cells by stimulating them with adjusted set of appropriate growth factors may result in efficient chondrogenic differentiation. However, those issues remain unresolved and require thorough investigation. Taken together, the application of MenSCs for chondrogenic differentiation can provide important information about cartilage function and repair potential and may possess significant regenerative value both as a tool for cartilage tissue engineering and for intra-articular cellular therapy based on stimulating paracrine effects. We conclude that these cells might become a realistic and attractive alternative for cartilage regeneration.
Acknowledgments
This work was funded by the European Social Fund according to the activity “Improvement of researchers' qualification by implementing world-class R&D projects” of Measure (No. 09.3.3-LMT-K-712) (grant application code: [09.3.3-LMT-K-712-01-0157], agreement no. DOTSUT-215).
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Authors' Contributions
G. Urbonaite and Z. Tachtamisevaite equally contributed to this manuscript.
References
- 1.Hass R., Kasper C., Böhm S., Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Communication and Signaling. 2011;9(1):p. 12. doi: 10.1186/1478-811X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim E.-J., Kim N., Cho S. G. The potential use of mesenchymal stem cells in hematopoietic stem cell transplantation. Experimental & Molecular Medicine. 2013;45(1, article e2) doi: 10.1038/emm.2013.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meng X., Ichim T. E., Zhong J., et al. Endometrial regenerative cells: a novel stem cell population. Journal of Translational Medicine. 2007;5(1):p. 57. doi: 10.1186/1479-5876-5-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heim M., Dudkiewicz I. Articular Cartilage Defects of the Knee. Springer; 2012. Articular cartilage defects of the knee: diagnosis and treatment; pp. 17–24. [DOI] [Google Scholar]
- 5.Mobasheri A., Matta C., Uzielienè I., Budd E., Martín-Vasallo P., Bernotiene E. The chondrocyte channelome: a narrative review. Joint Bone Spine. 2018 doi: 10.1016/j.jbspin.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 6.Bružauskaitė I., Bironaitė D., Bagdonas E., Bernotienė E. Scaffolds and cells for tissue regeneration: different scaffold pore sizes—different cell effects. Cytotechnology. 2016;68(3):355–369. doi: 10.1007/s10616-015-9895-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denkovskij J., Bagdonas E., Kusleviciute I., et al. Paracrine potential of the human adipose tissue-derived stem cells to modulate balance between matrix metalloproteinases and their inhibitors in the osteoarthritic cartilage in vitro. Stem Cells International. 2017;2017:13. doi: 10.1155/2017/9542702.9542702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borlongan C. V., Kaneko Y., Maki M., et al. Menstrual blood cells display stem cell–like phenotypic markers and exert neuroprotection following transplantation in experimental stroke. Stem Cells and Development. 2010;19(4):439–452. doi: 10.1089/scd.2009.0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tabatabaei F. S., Ai J. Mesenchymal endometrial stem/stromal cells for hard tissue engineering: a review of in vitro and in vivo evidence. Regenerative Medicine. 2017;12(8):983–995. doi: 10.2217/rme-2017-0029. [DOI] [PubMed] [Google Scholar]
- 10.Gargett C. E., Schwab K. E., Deane J. A. Endometrial stem/progenitor cells: the first 10 years. Human Reproduction Update. 2016;22(2):137–163. doi: 10.1093/humupd/dmv051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shamosi A., Mehrabani D., Azami M., et al. Differentiation of human endometrial stem cells into endothelial-like cells on gelatin/chitosan/bioglass nanofibrous scaffolds. Artificial Cells, Nanomedicine, and Biotechnology. 2016;45(1):163–173. doi: 10.3109/21691401.2016.1138493. [DOI] [PubMed] [Google Scholar]
- 12.Bayat N., Ebrahimi-Barough S., Ardakan M. M. M., et al. Differentiation of human endometrial stem cells into Schwann cells in fibrin hydrogel as 3D culture. Molecular Neurobiology. 2016;53(10):7170–7176. doi: 10.1007/s12035-015-9574-7. [DOI] [PubMed] [Google Scholar]
- 13.Lin J., Xiang D., Zhang J., Allickson J., Xiang C. Plasticity of human menstrual blood stem cells derived from the endometrium. Journal of Zhejiang University SCIENCE B. 2011;12(5):372–380. doi: 10.1631/jzus.B1100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hida N., Nishiyama N., Miyoshi S., et al. Novel cardiac precursor-like cells from human menstrual blood-derived mesenchymal cells. Stem Cells. 2008;26(7):1695–1704. doi: 10.1634/stemcells.2007-0826. [DOI] [PubMed] [Google Scholar]
- 15.Ikegami Y., Miyoshi S., Nishiyama N., et al. Serum-independent cardiomyogenic transdifferentiation in human endometrium-derived mesenchymal cells. Artificial Organs. 2010;34(4):280–288. doi: 10.1111/j.1525-1594.2009.00859.x. [DOI] [PubMed] [Google Scholar]
- 16.Azedi F., Kazemnejad S., Zarnani A. H., Soleimani M., Shojaei A., Arasteh S. Comparative capability of menstrual blood versus bone marrow derived stem cells in neural differentiation. Molecular Biology Reports. 2017;44(1):169–182. doi: 10.1007/s11033-016-4095-7. [DOI] [PubMed] [Google Scholar]
- 17.Faramarzi H., Mehrabani D., Fard M., et al. The potential of menstrual blood-derived stem cells in differentiation to epidermal lineage: a preliminary report. World Journal of Plastic Surgery. 2016;5(1):26–31. [PMC free article] [PubMed] [Google Scholar]
- 18.Chen J. Y., Mou X. Z., Du X. C., Xiang C. Comparative analysis of biological characteristics of adult mesenchymal stem cells with different tissue origins. Asian Pacific Journal of Tropical Medicine. 2015;8(9):739–746. doi: 10.1016/j.apjtm.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 19.Toyoda M., Cui C. H., Umezawa A. Myogenic transdifferentiation of menstrual blood-derived cells. Acta Myologica. 2007;26(3):176–178. [PMC free article] [PubMed] [Google Scholar]
- 20.Mou X., Lin J., Chen J. Y., et al. Menstrual blood-derived mesenchymal stem cells differentiate into functional hepatocyte-like cells. Journal of Zhejiang University SCIENCE B. 2013;14(11):961–972. doi: 10.1631/jzus.B1300081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Darzi S., Zarnani A. H., Jeddi-Tehrani M., et al. Osteogenic differentiation of stem cells derived from menstrual blood versus bone marrow in the presence of human platelet releasate. Tissue Engineering Part A. 2012;18(15-16):1720–1728. doi: 10.1089/ten.tea.2011.0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alcayaga-Miranda F., Cuenca J., Luz-Crawford P., et al. Characterization of menstrual stem cells: angiogenic effect, migration and hematopoietic stem cell support in comparison with bone marrow mesenchymal stem cells. Stem Cell Research & Therapy. 2015;6(1):p. 32. doi: 10.1186/s13287-015-0013-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kazemnejad S., Zarnani A. H., Khanmohammadi M., Mobini S. Stem Cell Nanotechnology. Vol. 1058. Totowa, NJ, USA: Humana Press; 2013. Chondrogenic differentiation of menstrual blood-derived stem cells on nanofibrous scaffolds; pp. 149–169. (Methods in Molecular Biology). [DOI] [PubMed] [Google Scholar]
- 24.Khanmohammadi M., Khanjani S., Bakhtyari M. S., et al. Proliferation and chondrogenic differentiation potential of menstrual blood- and bone marrow-derived stem cells in two-dimensional culture. International Journal of Hematology. 2012;95(5):484–493. doi: 10.1007/s12185-012-1067-0. [DOI] [PubMed] [Google Scholar]
- 25.Ren H., Sang Y., Zhang F., Liu Z., Qi N., Chen Y. Comparative analysis of human mesenchymal stem cells from umbilical cord, dental pulp, and menstrual blood as sources for cell therapy. Stem Cells International. 2016;2016:13. doi: 10.1155/2016/3516574.3516574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ponemone V., Gupta S., Suthar M. Emerging potential of cell based therapies for articular cartilage repair and regeneration. Advances in Tissue Engineering and Regenerative Medicine. 2017;3(2) doi: 10.15406/atroa.2017.03.00060. [DOI] [Google Scholar]
- 27.Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthritis and Cartilage. 2013;21(9):1145–1153. doi: 10.1016/j.joca.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arthritis Research UK. Osteoarthritis. Arthritis Foundation; 2012. [Google Scholar]
- 29.Hame S. L., Alexander R. A. Knee osteoarthritis in women. Current Reviews in Musculoskeletal Medicine. 2013;6(2):182–187. doi: 10.1007/s12178-013-9164-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mobasheri A., Rayman M. P., Gualillo O., Sellam J., Van Der Kraan P., Fearon U. The role of metabolism in the pathogenesis of osteoarthritis. Nature Reviews Rheumatology. 2017;13(5):302–311. doi: 10.1038/nrrheum.2017.50. [DOI] [PubMed] [Google Scholar]
- 31.Matta C., Zákány R., Mobasheri A. Voltage-dependent calcium channels in chondrocytes: roles in health and disease. Current Rheumatology Reports. 2015;17(7):p. 43. doi: 10.1007/s11926-015-0521-4. [DOI] [PubMed] [Google Scholar]
- 32.Tang L., Gamal el-Din T. M., Payandeh J., et al. Structural basis for Ca2+ selectivity of a voltage-gated calcium channel. Nature. 2014;505(7481):56–61. doi: 10.1038/nature12775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang M., Yuan Z., Ma N., et al. Advances and prospects in stem cells for cartilage regeneration. Stem Cells International. 2017;2017:16. doi: 10.1155/2017/4130607.4130607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heo J. S., Choi Y., Kim H. S., Kim H. O. Comparison of molecular profiles of human mesenchymal stem cells derived from bone marrow, umbilical cord blood, placenta and adipose tissue. International Journal of Molecular Medicine. 2016;37(1):115–125. doi: 10.3892/ijmm.2015.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshiya S., Dhawan A. Cartilage repair techniques in the knee: stem cell therapies. Current Reviews in Musculoskeletal Medicine. 2015;8(4):457–466. doi: 10.1007/s12178-015-9302-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Somoza R. A., Welter J. F., Correa D., Caplan A. I. Chondrogenic differentiation of mesenchymal stem cells: challenges and unfulfilled expectations. Tissue Engineering Part B: Reviews. 2014;20(6):596–608. doi: 10.1089/ten.teb.2013.0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van der Kraan P. M., van den Berg W. B. Chondrocyte hypertrophy and osteoarthritis: role in initiation and progression of cartilage degeneration? Osteoarthritis and Cartilage. 2012;20(3):223–232. doi: 10.1016/j.joca.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 38.Danišovič Ľ., Varga I., Polák Š. Growth factors and chondrogenic differentiation of mesenchymal stem cells. Tissue and Cell. 2012;44(2):69–73. doi: 10.1016/j.tice.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 39.Derynck R., Piek E., Schneider R. A., Choy L., Alliston T. TGF-β Family Signaling in Mesenchymal Differentiation. Cold Spring Harbor Laboratory Press; 2008. [Google Scholar]
- 40.Shu C., Smith S. M., Little C. B., Melrose J. Use of FGF-2 and FGF-18 to direct bone marrow stromal stem cells to chondrogenic and osteogenic lineages. Future Science OA. 2016;2(4, article FSO142) doi: 10.4155/fsoa-2016-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moon R. T., Bowerman B., Boutros M., Perrimon N. The promise and perils of Wnt signaling through β-catenin. Science. 2002;296(5573):1644–1646. doi: 10.1126/science.1071549. [DOI] [PubMed] [Google Scholar]
- 42.Pizette S., Niswander L. BMPs are required at two steps of limb chondrogenesis: formation of prechondrogenic condensations and their differentiation into chondrocytes. Developmental Biology. 2000;219(2):237–249. doi: 10.1006/dbio.2000.9610. [DOI] [PubMed] [Google Scholar]
- 43.Retting K. N., Song B., Yoon B. S., Lyons K. M. BMP canonical Smad signaling through Smad1 and Smad5 is required for endochondral bone formation. Development. 2009;136(7):1093–1104. doi: 10.1242/dev.029926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taipaleenmäki H. Factors Regulating Chondrogenic Differentiation. Annales Universitatis TurkuensisIssue: Medica-Odontologica; 2010. [Google Scholar]
- 45.Ito T., Sawada R., Fujiwara Y., Tsuchiya T. FGF-2 increases osteogenic and chondrogenic differentiation potentials of human mesenchymal stem cells by inactivation of TGF-β signaling. Cytotechnology. 2008;56(1):1–7. doi: 10.1007/s10616-007-9092-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu D. A., Han J., Kim B. S. Stimulation of chondrogenic differentiation of mesenchymal stem cells. International Journal of Stem Cells. 2012;5(1):16–22. doi: 10.15283/ijsc.2012.5.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lefebvre V., Li P., De Crombrugghe B. A new long form of Sox5 (L-Sox5), Sox6 and Sox9 are coexpressed in chondrogenesis and cooperatively activate the type II collagen gene. The EMBO Journal. 1998;17(19):5718–5733. doi: 10.1093/emboj/17.19.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gadjanski I., Spiller K., Vunjak-Novakovic G. Time-dependent processes in stem cell-based tissue engineering of articular cartilage. Stem Cell Reviews and Reports. 2012;8(3):863–881. doi: 10.1007/s12015-011-9328-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Girkontaite I., Frischholz S., Lammi P., et al. Immunolocalization of type X collagen in normal fetal and adult osteoarthritic cartilage with monoclonal antibodies. Matrix Biology. 1996;15(4):231–238. doi: 10.1016/S0945-053X(96)90114-6. [DOI] [PubMed] [Google Scholar]
- 50.Mitsugi S., Ariyoshi W., Okinaga T., et al. Mechanisms involved in inhibition of chondrogenesis by activin-A. Biochemical and Biophysical Research Communications. 2012;420(2):380–384. doi: 10.1016/j.bbrc.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 51.Majumdar M. K., Thiede M. A., Mosca J. D., Moorman M., Gerson S. L. Phenotypic and functional comparison of cultures of marrow-derived mesenchymal stem cells (MSCs) and stromal cells. Journal of Cellular Physiology. 1998;176(1):57–66. doi: 10.1002/(SICI)1097-4652(199807)176:1<57::AID-JCP7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 52.Nikoo S., Ebtekar M., Jeddi-Tehrani M., et al. Menstrual blood-derived stromal stem cells from women with and without endometriosis reveal different phenotypic and functional characteristics. Molecular Human Reproduction. 2014;20(9):905–918. doi: 10.1093/molehr/gau044. [DOI] [PubMed] [Google Scholar]
- 53.Du X., Yuan Q., Qu Y., Zhou Y., Bei J. Endometrial mesenchymal stem cells isolated from menstrual blood by adherence. Stem Cells International. 2016;2016:8. doi: 10.1155/2016/3573846.3573846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xia Q., Zhu S., Wu Y., et al. Intra‐articular transplantation of atsttrin‐transduced mesenchymal stem cells ameliorate osteoarthritis development. Stem Cells Translational Medicine. 2015;4(5):523–531. doi: 10.5966/sctm.2014-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Djouad F., Jackson W. M., Bobick B. E., et al. Activin A expression regulates multipotency of mesenchymal progenitor cells. Stem Cell Research & Therapy. 2010;1(2):p. 11. doi: 10.1186/scrt11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peran M., Ruiz S., Kwiatkowski W., et al. Activin/BMP2 chimeric ligands direct adipose-derived stem cells to chondrogenic differentiation. Stem Cell Research. 2013;10(3):464–476. doi: 10.1016/j.scr.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 57.Alexander S., Watt F., Sawaji Y., Hermansson M., Saklatvala J. Activin A is an anticatabolic autocrine cytokine in articular cartilage whose production is controlled by fibroblast growth factor 2 and NF-κB. Arthritis & Rheumatism. 2007;56(11):3715–3725. doi: 10.1002/art.22953. [DOI] [PubMed] [Google Scholar]
- 58.Wijayarathna R., de Kretser D. M. Activins in reproductive biology and beyond. Human Reproduction Update. 2016;22(3):342–357. doi: 10.1093/humupd/dmv058. [DOI] [PubMed] [Google Scholar]
- 59.Wolff E. F., Wolff A. B., Hongling du, Taylor H. S. Demonstration of multipotent stem cells in the adult human endometrium by in vitro chondrogenesis. Reproductive Sciences. 2007;14(6):524–533. doi: 10.1177/1933719107306896. [DOI] [PubMed] [Google Scholar]
- 60.Patel A. N., Park E., Kuzman M., Benetti F., Silva F. J., Allickson J. G. Multipotent menstrual blood stromal stem cells: isolation, characterization, and differentiation. Cell Transplantation. 2008;17(3):303–311. doi: 10.3727/096368908784153922. [DOI] [PubMed] [Google Scholar]
- 61.Rossignoli F., Caselli A., Grisendi G., et al. Isolation, characterization, and transduction of endometrial decidual tissue multipotent mesenchymal stromal/stem cells from menstrual blood. BioMed Research International. 2013;2013:14. doi: 10.1155/2013/901821.901821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khanjani S., Khanmohammadi M., Zarnani A. H., et al. Comparative evaluation of differentiation potential of menstrual blood- versus bone marrow- derived stem cells into hepatocyte-like cells. PLoS One. 2014;9(2, article e86075) doi: 10.1371/journal.pone.0086075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tang Y., Liu L., Wang P., Chen D., Wu Z., Tang C. Periostin promotes migration and osteogenic differentiation of human periodontal ligament mesenchymal stem cells via the Jun amino-terminal kinases (JNK) pathway under inflammatory conditions. Cell Proliferation. 2017;50(6, article e12369) doi: 10.1111/cpr.12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Coutu D. L., Wu J. H., Monette A., Rivard G. É., Blostein M. D., Galipeau J. Periostin, a member of a novel family of vitamin K-dependent proteins, is expressed by mesenchymal stromal cells. The Journal of Biological Chemistry. 2008;283(26):17991–18001. doi: 10.1074/jbc.M708029200. [DOI] [PubMed] [Google Scholar]
- 65.Zhou N., Li Q., Lin X., et al. BMP2 induces chondrogenic differentiation, osteogenic differentiation and endochondral ossification in stem cells. Cell and Tissue Research. 2016;366(1):101–111. doi: 10.1007/s00441-016-2403-0. [DOI] [PubMed] [Google Scholar]
- 66.Fu H. L., Diao Z. Y., Shao L., Yang D. P. BMP-2 promotes chondrogenesis of rat adipose-derived stem cells by using a lentiviral system. Genetics and Molecular Research. 2014;13(4):8620–8631. doi: 10.4238/2014.October.27.1. [DOI] [PubMed] [Google Scholar]
- 67.Murphy M. K., Huey D. J., Hu J. C., Athanasiou K. A. TGF-β1, GDF-5, and BMP-2 stimulation induces chondrogenesis in expanded human articular chondrocytes and marrow-derived stromal cells. Stem Cells. 2015;33(3):762–773. doi: 10.1002/stem.1890. [DOI] [PubMed] [Google Scholar]