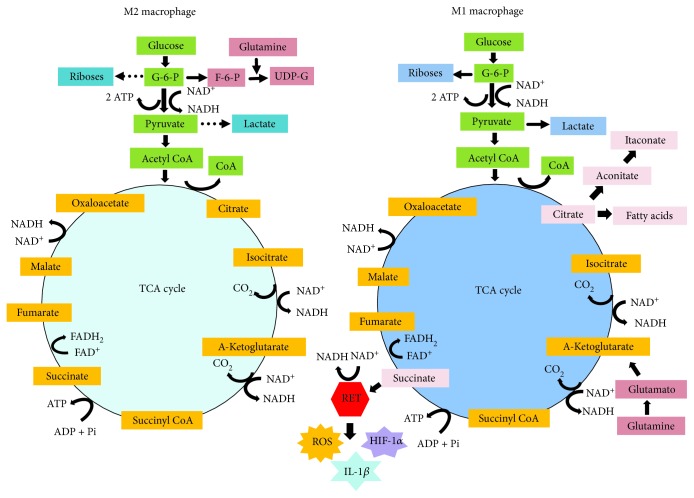
Figure 2.
Metabolic pathways supporting macrophage reprogramming in either M2 or M1 phenotype. IL-4-stimulated M2 macrophage utilizes glycolysis, TCA, and mitochondrial oxidative phosphorylation (OXPHOS) to generate NADPH and ATP for energy. G-6-P and glutamine are used for generation of UDP-Glc-Nac required for receptors and protein glycosylation. LPS-stimulated M1 macrophage produces G-6-P that is reduced to pyruvate, in parallel, NAD+ is reduced to NADPH, and 2 ATP molecules are produced. In hypoxia, pyruvate is reduced to lactate, restoring NAD+ to glycolysis recycle, and thus, OXPHOX is uncoupled to glycolysis. TCA cycle in LPS-stimulated M1 macrophageis deviated after citrate and after succinate. Citrate is used for the syntheses of NO, ROS, and prostaglandins. Citrate is used by the enzyme immune-responsive gene 1 (IRG1) to generate itaconate that acts as an endogenous SDH inhibitor and also as antimicrobial. Succinate is oxidized by SDH and subsequently used for production of ROS from a complex 1 via reverse electron transport (RET). Succinate activates HIF-1α which in turn increases the transcription of IL-1β. Glutaminolysis provides NADPH for generation of ROS. SDH: succinate dehydrogenase; UDP-Glc-Nac: uridine diphosphate N-acetylglucosamine; NO: nitric oxide; ROS: reactive oxygen species. Arrows indicated the direction of reaction and products.