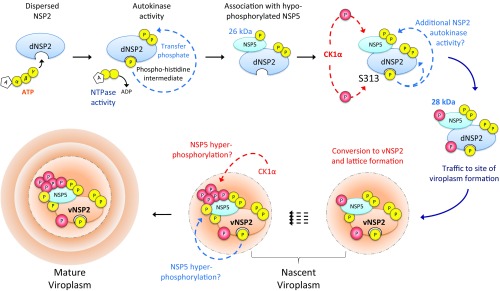
Fig. 7.
Model of phosphorylation cascade and viroplasm assembly. Based on our studies we hypothesize that dNSP2 autophosphorylates via the NTPase activity, creating a transient phosphohistidine intermediate. Autophosphorylated NSP2 remains dispersed in the cytoplasm and associates with hypophosphorylated NSP5. CK1α then phosphorylates NSP2 on S313. CK1α may phosphorylate NSP5, leading to the appearance of the 28-kDa isoform. NSP2 may also phosphorylate NSP5. NSP2 phospho-S313 complexed with NSP5 traffics to the site of viroplasm formation and undergoes further conformational changes, by an unknown process, into vNSP2. NSP5 is rapidly hyperphosphorylated to the 32- to 35-kDa isoforms by CK1α and/or NSP2 to remain associated with vNSP2. Repetition of this process leads to growth and maturation of the viroplasm.