Fig. 6.
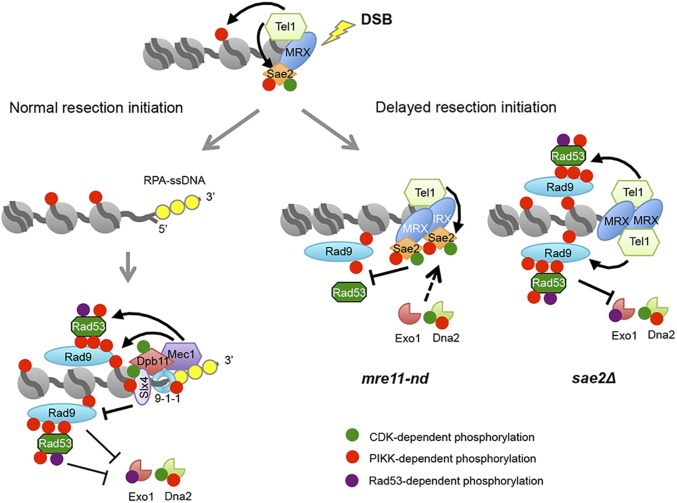
Sae2 controls short- and long-range resection pathways. Normally, resection initiation by Mre11 and Sae2 is fast resulting in low dwell time of Tel1 at DSBs, and consequently low activation of Tel1 kinase (for simplicity, only one side of a DSB is shown). After resection initiation, Mec1-Ddc2 is recruited to ssDNA overhangs, phosphorylating Rad9 and Rad53 to slow down resection by Dna2-Sgs1 and Exo1. Slx4-Rtt107 competes with Rad9 for Dpb11 binding to dampen the checkpoint and, consequently, increase extensive resection. When resection initiation is compromised by the mre11-nd mutation, MRX, Tel1, and Sae2 accumulate at DSBs and Sae2 competes with other Tel1 substrates for phosphorylation, reducing Rad9 binding, Rad53 activation, and allowing resection by Dna2-Sgs1 and Exo1. In the absence of Sae2, Tel1 is hyperactivated, causing increased Rad9 binding and Rad53 activation, thereby diminishing resection by Dna2-Sgs1 and Exo1.