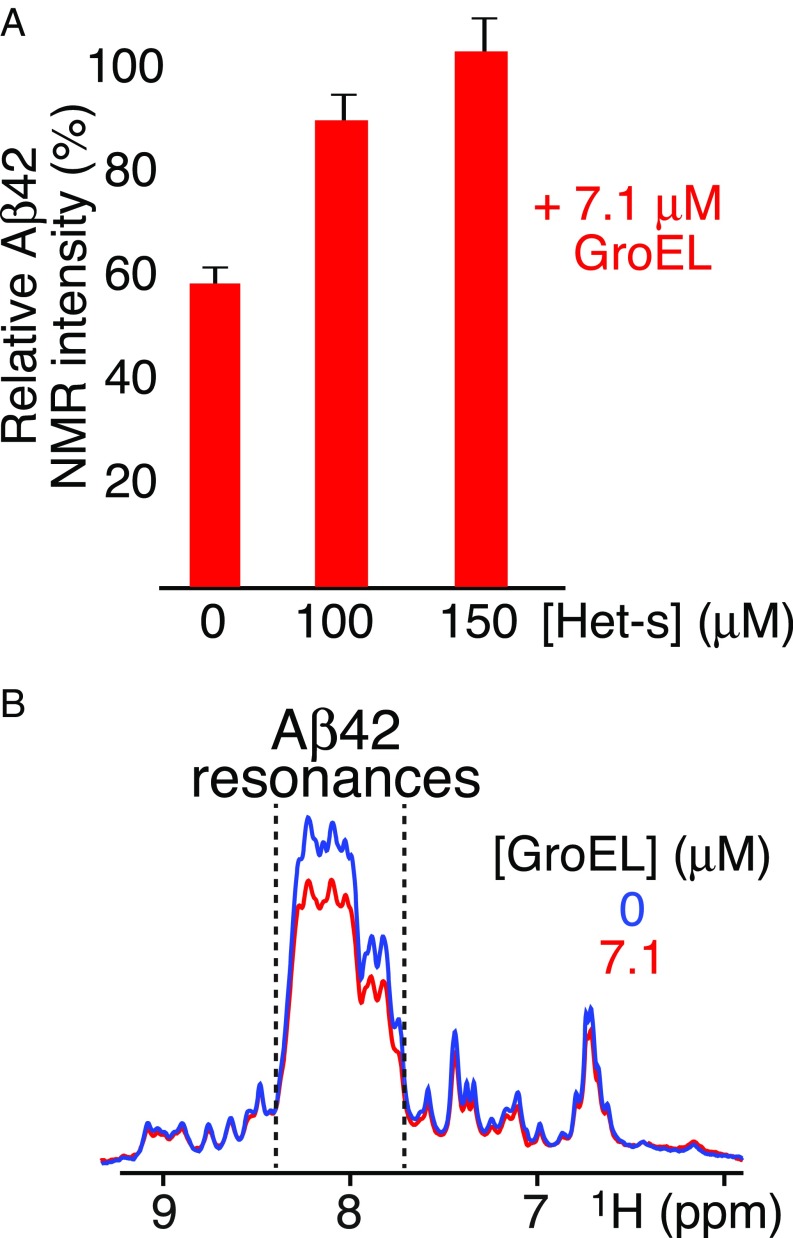
Fig. 4.
Effect of addition of Het-s or fusion of Aβ42 with the B1 domain of protein G (GB1) upon GroEL binding. (A) Intensity of the backbone amide envelope of 100 μM 15N-labeled Aβ42 immediately after dissolving the peptide (i.e., time point zero) in the presence of GroEL (7.1 μM) relative to that in absence of GroEL. The backbone envelope intensity is obtained from the Fourier transform of the FID of the first t1 increment of a 1H–15N correlation experiment. Addition of Het-s displaces GroEL-bound Aβ42, resulting in restoration in the intensity of the amide proton envelope of the Aβ42 spectrum to the same level as that seen in the absence of GroEL. (B) First Fourier-transformed t1 increment of a 1H–15N correlation experiment of the 15N-labeled GB1–Aβ42 fusion protein (100 μM, directly after SEC) in the absence (blue) and presence of 7.1 μM GroEL (red). A decrease in intensity of ∼20% is seen between 7.7 and 8.4 ppm (delineated by the dashed lines) which contains all of the backbone amide resonances of Aβ42 (Fig. 3A), typical of a random coil, as well as some of GB1, but not downfield of 8.4 ppm or upfield of 7.7 ppm where only Asn and Gln side chain amido (below 7.5 ppm) and GB1 backbone amide resonances (with spectral dispersion characteristic of a folded globular domain) are present, indicating that the fusion protein binds as a monomer and that only the Aβ42 moiety of the fusion protein interacts with GroEL.