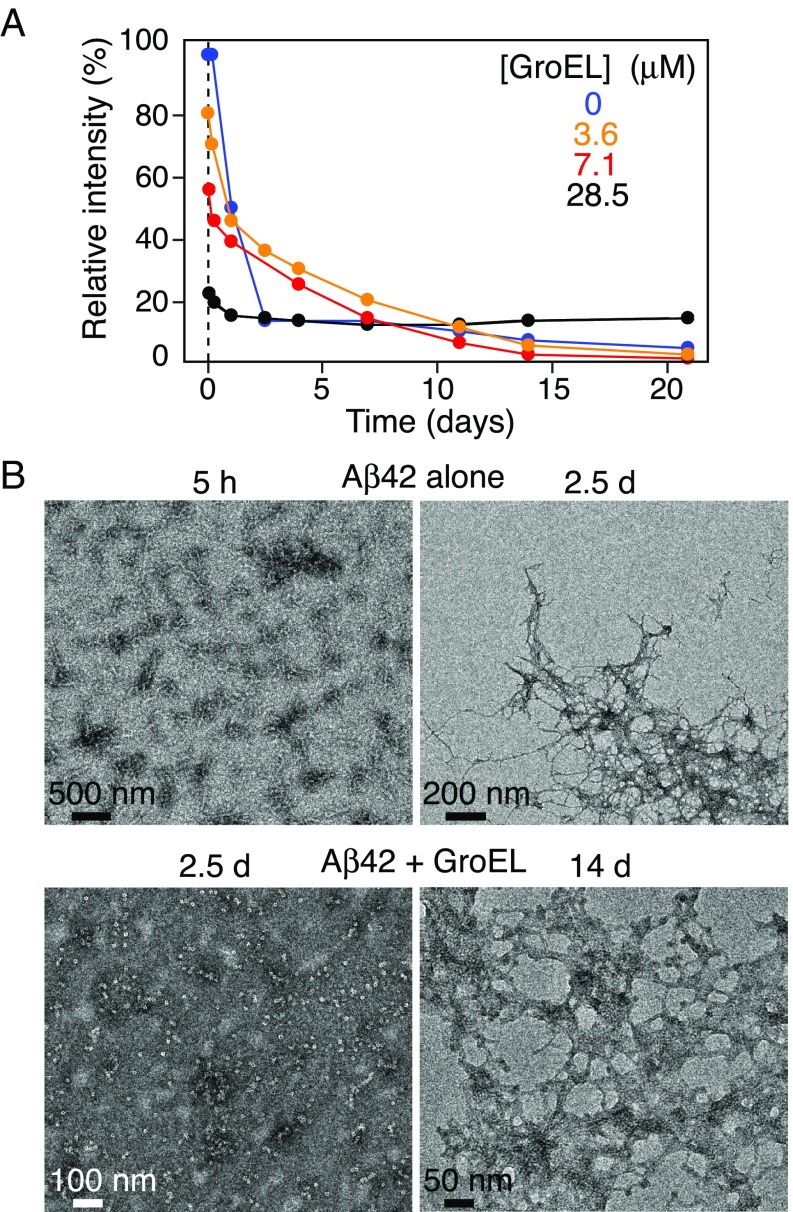
Fig. 8.
Time dependence of Aβ42 aggregation in the presence and absence of GroEL. (A) Relative intensity of the backbone amide proton envelope of 50 μM 15N-labeled Aβ42 obtained from the first t1 increment of a 1H–15N correlation experiment recorded as a function of time (up to 21 d) in the presence of various concentrations of GroEL (ranging from 0 to 28.5 μM). (B) Electron micrographs of Aβ42 over time in the absence (Top row) and presence (Bottom row) of GroEL. The Bottom Left image obtained after 2.5 d in the presence of 28.6 μM GroEL shows no evidence of fibrils; significant fibril formation is apparent in the Bottom Right image after 14-d incubation with 7.1 µM GroEL. Serial EM and AFM images over a period of 0–14 d and 0–4 wk, respectively, are shown in SI Appendix, Fig. S11. The aggregation was allowed to proceed at room temperature, without shaking.