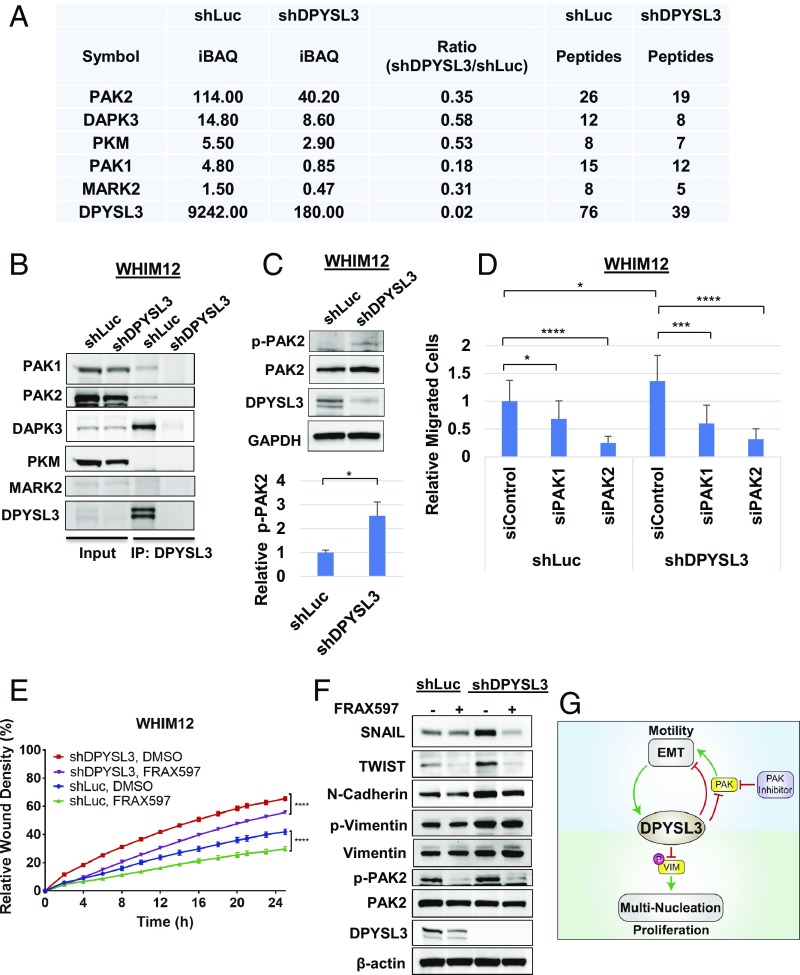
Fig. 7.
PAK2 interacts with DPYSL3 to modulate migration. (A) Table depicting DPYSL3 interacting kinases pulled down by DPYSL3 IP/mass spectrometry data from stable WHIM12 shLuc and shDPYSL3 cell lines. Accompanying information is presented in Dataset S3. iBAQ, intensity-based absolute quantification. (B) Western blots from inputs and DPYSL3 immunoprecipitated lysates from stable WHIM12 shLuc and shDPYSL3 cell lines. (C, Upper) Western blots from stable WHIM12 shLuc and shDPYSL3 cells. (C, Lower) Phospho-PAK2 (p-PAK2) from three independent experiments ± SEM. *P < 0.05 (Student t test). (D) Quantification of cell migration from stable WHIM12 cells transfected with siRNA after transwell migration assay relative to shLuc cells transfected with siControl. Data were shown as the averages from three independent experiments ± SEM. *P < 0.05; ***P < 0.001; ****P < 0.0001 (ANOVA with Tukey’s multiple comparisons test). (E) Quantification of cell migration as relative wound densities of stable WHIM12 shLuc and shDPYSL3 cells treated with DMSO or FRAX597 (1 M) at time points postwounding. P value was determined by ANOVA. ****P < 0.0001 (Bonferroni’s multiple comparisons test). (F) WHIM12 shLuc and shDPYSL3 cells were treated with DMSO or FRAX597 (1 M) for 36 h. Western blotting was performed with the indicated antibodies in WHIM12 shLuc and WHIM12 shDPYSL3 with or without FRAX597 treatment. (G) Working model of DPYSL3 as a multifunctional signaling scaffold. DPYSL3 is induced during EMT and provides a negative feedback on EMT. PAK inhibition suppresses cell migration in CLOW breast cancer. DPYLS3 promotes proliferation via positive regulation of the mitotic spindle. Loss of DPYSL3 induces vimentin phosphorylation leading to cytokinetic failure and multinucleation, thus impairing proliferation.