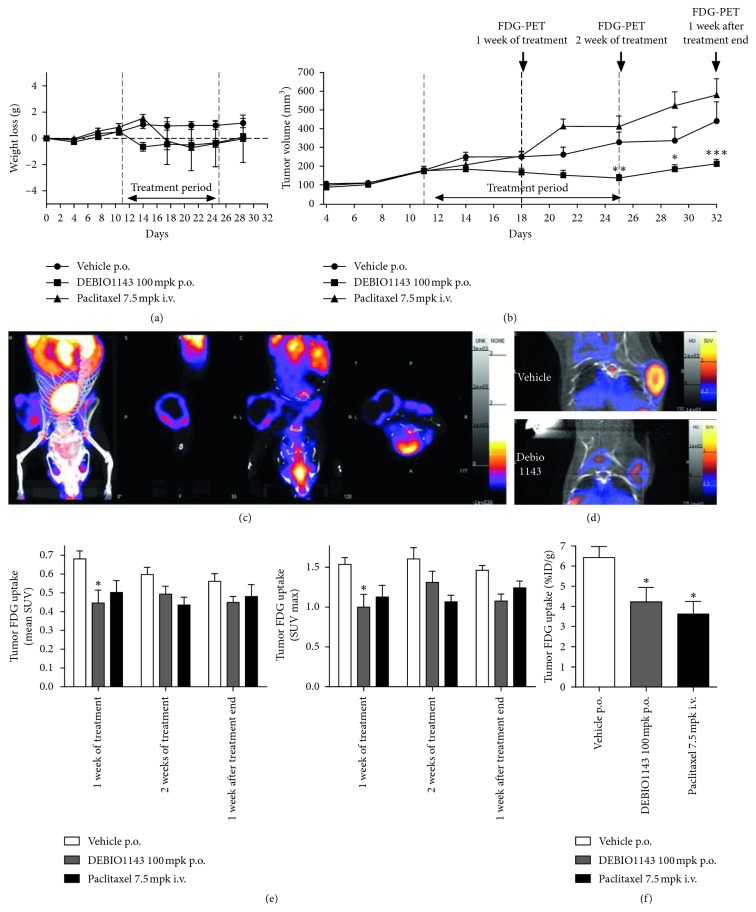
Figure 3.
In vivo evaluation of the antitumor activity of D1143 by [18F]-FDG PET-CT. (a) Weight loss (g) monitoring of tumor-bearing SCID mice receiving D1143, paclitaxel, or vehicle as control. Treatment started at D11 and ended at D25. Mice were sacrificed at D32. Results are presented as mean ± SEM; n=8. (b) Tumor volume (mm3) of tumor-bearing SCID mice receiving D1143, paclitaxel, or vehicle as control. Treatment started at D11 and ended at D25. Results are presented as mean ± SEM; n=8; ∗∗ p < 0.01, ∗∗∗ p < 0.01. (c) Representative [18F]-FDG PET-CT picture of tumor-bearing SCID mice (tumor in the right shoulder) receiving vehicle at D32. (d) Representative [18F]-FDG PET-CT picture of tumor-bearing SCID mice (tumor in the right shoulder) receiving vehicle or D1143 at D32. (e) Tumor [18F]-FDG uptake in SCID mice receiving vehicle, D1143, or paclitaxel. Measures have been performed at D18 (1 week of treatment), D25 (2 weeks of treatment), and D32 (1 week after treatment). Results are expressed as mean SUV (left panel) and SUV max (right panel). Results are presented as mean ± SEM; n=8, ∗ p < 0.05. (f) Gamma counting of [18F]-FDG in tumors of SCID mice receiving paclitaxel (iv), D1143 (po), or vehicle as control (%ID/g). Results are presented as mean ± SEM; n=8; ∗ p < 0.05.