Abstract
Background:
Administration of systemic antibiotics may implement persuasive treatment effect for chronic periodontitis by intending tissue-invasive bacteria in addition to accustomed nonsurgical periodontal therapy (NSPT).
Aims:
The aim of this study was to assess the ancillary effects of oral clarithromycin (CLM) along with NSPT for chronic periodontitis.
Materials and Methods:
Thirty periodontitis patients were randomly divided into two equal groups in this double-blind, randomized, parallel group, and active-controlled trial: test group – scaling and root planning (SRP) plus CLM (500 mg thrice daily for 7 days, orally) was given, and control group – only SRP was done. Clinical analysis, such as gingival index (GI), probing depth (PD), and clinical attachment loss (CAL), were taken at baseline, 3 months, and 6-month intervals for both groups. Subgingival plaque samples were cultured for periodontopathic organisms. Immunological parameter C-reactive protein (CRP) levels were estimated.
Results:
SPSS version 14 was used for statistical analysis. The intragroup comparison showed a significant reduction in the mean scores of all the parameters from baseline to 6 months. The intergroup comparison showed a statistically significant reduction of PD from baseline to 3 months (P < 0.001). GI, CAL, and CRP levels were also reduced but not statistically significant. The mean colony-forming units (CFU) of Aggregatibacter actinomycetemcomitans (Aa) and Porphyromonas gingivalis (Pg) showed a statistically significant reduction from baseline to 3 months only in the test group (P = 0.042) and (P = 0.046), respectively. There was no statistically significant reduction of Aa and Pg at 6 months.
Conclusions:
CLM conceivably accepted as an addendum to NSPT for a shorter period.
Keywords: Antimicrobial agents, colony-forming units, clarithromycin, microbial colonization, periodontitis, scaling and root planing
INTRODUCTION
Periodontal diseases occur with a greater incidence and prevalence and are a major oral health problem worldwide. This disease is caused by pathogenic microbial species involving the subgingival niche, which threatens the loss of teeth. A multitude of bacterial geniuses inhabits in the periodontal pockets due to the dampish, perspiring, beneficial, and anaerobic environment. Dental plaque is a complex structure; classic example of biofilm which contains abundant bacterial species and is tightly attached to the tooth surface. The pathogenic and invading nature of the complex bacterial communities may imprecise treatment modalities. The ramification of antimicrobial therapies was intended to overwhelming this bacterial capability.[1]
Indeed, at the fifth European Workshop of Periodontology, it was consummated that “dental plaque displays properties that are typical of biofilms and microbial communities in general, a clinical repercussion of which is a diminished perceptivity to antimicrobial agents as well as pathogenic symbiosis”[2] The abundance and diversity of periodontal pocket microorganisms rely on assorted circumstances, in addition to the effectiveness of oral hygiene measures, pocket depth, degree of gingival inflammation, flow of gingival crevicular fluid, type of communicating microbes, transmission rate of microbes from other individuals, and the antimicrobial efficacy of the host immune response.[3]
The specific properties of biofilm and various factors such as root concavities, furcation involvement, and other anatomical aberrations make subgingival periodontal pathogens more troublesome to target, which necessitated the advancement of procedures to treat the subgingival microflora, such as the use of chemotherapeutic agents which provide an additional benefit to the conventional therapy by their intrinsic activity. Invasive pathogens can be eliminated by introducing an appropriate antimicrobial agent into invaded host cells.[4]
The question of whether scaling and root planing (SRP) is string along by an antimicrobial agent clarithromycin (CLM), as a supporting or addendum to treatment, consequence in enhanced payoff as compared to SRP alone was probed in this study.
MATERIALS AND METHODS
The Institutional Ethical Board approval was taken before the study (ref no 0055); and also registered under https://clinicaltrials.gov (NCT02359721). This double-blind randomized, parallel group and active-controlled trial compared 50 patients, who attended the Outpatient Department of Periodontics, were screened. All 50 patients met with inclusion criteria (mentioned below) of which only 30 patients have given their acquiescence to be a participant in the study. All the patients’ age ranged from 30 to 50 years old diagnosed with chronic periodontitis (according to the revised periodontal disease classification)[5] were randomly prorated into two groups, control group (15) and test (15) group [Figure 1].
Figure 1.
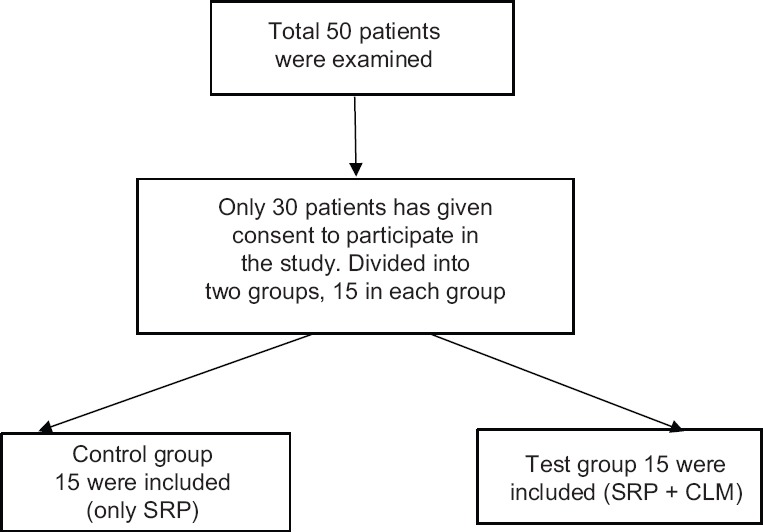
Blood agar plates with Porphyromonas gingivalis black colonies
The baseline and follow-up of clinical specifications, gingival index (GI), probing depth (PD), and Clinical Attachment Level (CAL) were evaluated by one operator who was not aware of the groups. All the participants were treated by another operator and kept blinded of the baseline and follow-up readings of the results. Subgingival samples were collected for microbiological analysis, and blood samples were collected for immunological assay in both groups. All the samples were collected in a prefumigated theater, and strict aseptic methods followed. No adverse effects were found throughout the study.
Inclusion criteria
Pocket PD >5 mm. clinical attachment level (CAL) >3 mm,[5] and no other systemic health problems were included in this study.
Exclusion criteria
Exclusion criteria include those who have undergone periodontal therapy within 3 months earlier to the study, present/past smokers, any antibiotics/any other drugs used within 3 months earlier to the study, pregnant women and lactating mothers, uncompromising and subnormal patients, suffering from any other systemic diseases, any drug allergy/other allergies, and patients who are perpetuating recall maintenance visits.
Control group
Only SRP was done; and clinical, microbiological, and immunological parameters assessed at baseline, 3 months, and 6 months.
Test group
CLM (500 mg three times per day (t.i.d) for 7 days) has given along with SRP, clinical, microbiological, and immunological parameters assessed at baseline, 3 and 6 months.
Clinical parameters: all the periodontal parameters were measured manually with William's graduated periodontal probe. GI – Loe and Silness[6] GI was taken; PD and CAL were measured; and comparative consecutive scores were given in both groups.
Microbiological analysis
Subgingival plaque samples were collected from both groups; microbial colony-forming units (CFU) of “Aa and Pg” were assessed.
Procedure
Subgingival plaque samples were collected from the deep periodontal pocket with a sterilized curette. The samples were transferred aseptically to a transport medium containing; Robertson's cooked meat broth. Sealed immediately to create strict anaerobic condition, incubated at 37°C for 24 h. After incubation, turbid appearance indicated the presence/growth of the culture. Subculture was made on blood agar (BA) (5% sheep blood), and brain heart infusion agar (0.8 g) supplemented with Vitamin K (0.1 mg/L) and hemin (1 g/ml). These plates were incubated at 37°C for 72 h. After incubation, the culture was stained with Gram staining. At this stage, on BA plates, Pg black colonies were identified [Figure 2]. The samples were cultured on chocolate agar (CA) incubated for 3 days with 5% carbon dioxide at 37°C. Ag was identified as white star shaped [Figure 3]. Microscopically, further suspected colonies were identified with Gram staining. Finally, computations were transmuting in colony-forming units per millimeter of the authentic sample.
Figure 2.
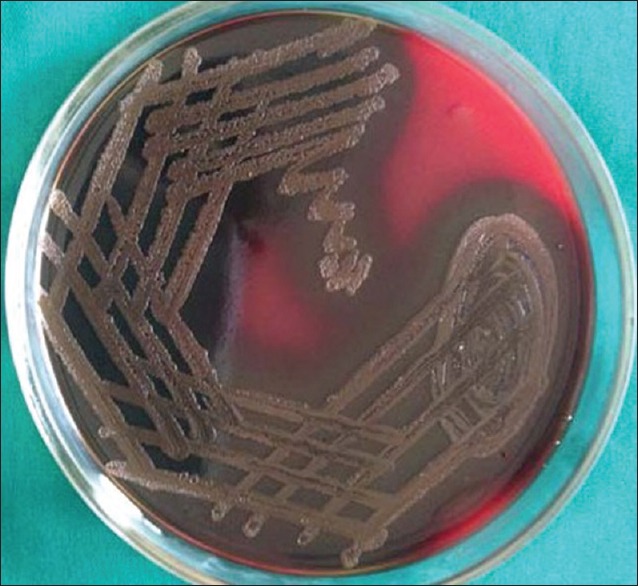
Patients’ selection criteria
Figure 3.
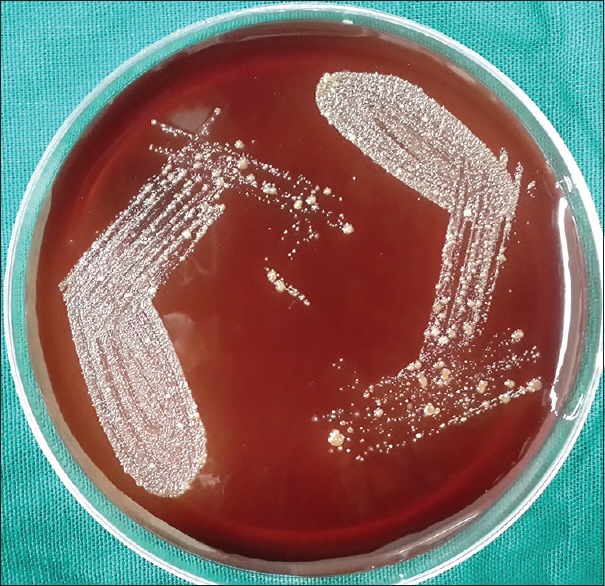
Chocolate agar plates with Aggregatibacter actinomycetemcomitans white star-shaped colonies
Immunological assay
C-reactive protein (CRP) was assessed with alpha diagnostic international human CRP ELISA Kit. A volume of 5 ml of peripheral venous blood was drawn from brachial plexus through vein puncture method. Whole blood was collected in test tubes and centrifuged at 1000 ×g for 10 min, in a centrifuge; the serum was obtained. With the help of Pasteur pipette, liquid component (serum) was transferred into clean polypropylene tubes immediately. These tubes were stored at 20°C until used.
Procedure
Human CRP ELISA kit is counted on concurrent binding of human CRP from constituent of two antibodies, one debilitate on the microtitter well plates, and other banded to the enzyme horseradish peroxidase. Subsequently, completion of the ablutionary action, colors developed by adding a chromogenic substrate. The enzymatic response (color) is directly proportional to the quantity of CRP existing in the sampling. Count-up stopping solution wraps up the domino effect. Acquaintance was then deliberated on a microtitter well ELISA bibliomaniac at 450 nm and the aggregation of CRP in constituent and control was read off the standard curve.
RESULTS
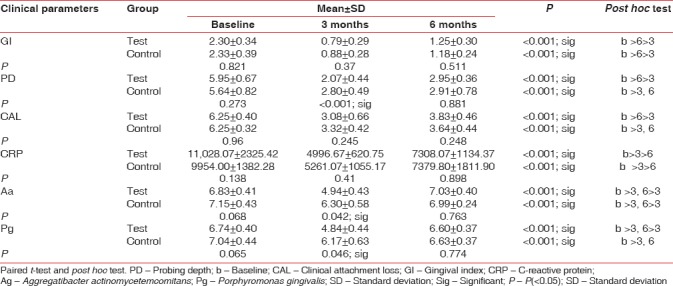
All the statistical analysis were performed using SPSS version 14 (IBM, Chicago, IL, USA). <0.05 was considered statistically significant. Independent sample t-test was performed to compare the mean values between the groups. Intragroup variations were done using repeated measures ANOVA with post hoc Bonferroni test. Mean percentage changes were correlated between groups using independent sample t-test. GI scores in test and control groups were 2.30 and 2.33 at baseline, 0.79 and 0.88 at 3 months, and 1.25 and 1.18 at 6 months’ intervals. Depreciation of values was seen at 3 months; however, this depreciation was not statistically significant. PD scores in test and control groups were 5.95 and 5.64 at baseline, 2.07 and 2.80 at 3 months, and 2.95 and 2.91 at 6 months intervals. A significant diminution was seen at 3 months in both groups (P < 0.001). CAL in test and control groups was 6.25 and 6.25 at baseline, 3.08 and 3.32 at 3 months, and 3.83 and 3.64 at 6 months’ intervals. Reduction was seen at 3 months and 6 months but statistically not significant Table 1 and Graph 1].
Table 1.
Intragroup and intergroup comparisons of all the parameters at baseline, 3 months, and 6 months
Graph 1.
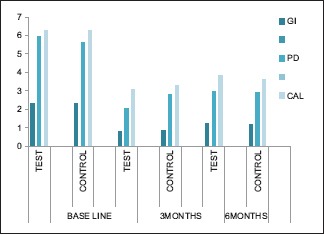
Periodontal parameters (gingival index, probing depth, and CAL) at 0, 3, and 6 months’ intervals in both groups
CRP levels were reduced convincingly from baseline to 3 months, and increased levels were seen from 3 to 6 months in test and control groups, although not up to the baseline [Table 1 and Graph 2].
Graph 2.
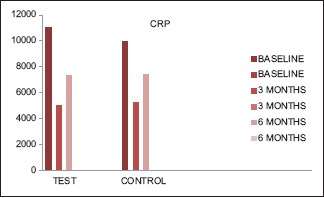
Immunological parameters (C-reactive protein) at 0, 3, and 6 months’ intervals in both the groups
Total comparable counts in a log of CFU of Aa in test and control groups were 6.83 and 7.15 at baseline, 4.94 and 6.30 at 3 months, and 7.03 and 6.99 at 6 months lacuna. A significant decline was seen at 3 months in test and control groups (P = 0.042). Straight up the consequence at 6 months in the test group. CFU of Pg in test and control groups was 6.74 and 7.04 at baseline, 4.84 and 6.17 at 3 months, and 6.60 and 6.63 at 6 months’ lacuna. A significant decrease was seen at 3 months in test and control groups (P = 0.046). Ricochet repercussion was seen in both groups at 6 months [Table 1 and Graph 3]. Sneaking suspicion of these results, all the parameters devaluated from baseline to 3 months; however, at 6 months hark back, near-at-hand to baseline. Among these entire variables, a significant markdown was seen, particularly in PD, Aa, and Pg, at 3 months.
Graph 3.
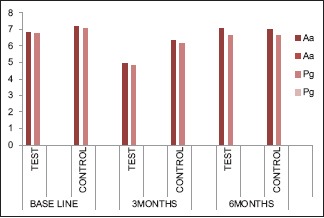
Microbial parameters Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis levels at 0, 3, and 6 months’ intervals in both the groups
DISCUSSION
Systemic antimicrobials used as an adjuvant therapy in the treatment of periodontitis was proclaimed in many systematic reviews, few were discussed at European world Workshop by Herrera et al.[7] and Haffajee et al.[8] they have concluded that in specific clinical situations, such as in patients with deep pockets, patients with accelerating or progressive disease, or with specific microbiological profiles, this antimicrobial therapy corroborative to SRP could be clinically admissible. Garcia et al.[9] described the use of systemic antibiotics in concurrence with SRP not only favors to improve the clinical outcomes but also helps in successful periodontal treatment.
Among many acute phase proteins, chronic periodontal disease can be lump together with an added on provocative reaction, established as raised up CRP elevation. CRP is the one with raised plasma and serum levels during the specific and nonspecific responses to a wide variety of diseases. Provincial diseases culminate in elevated inflammation, and tissue loss in the periodontium gives rise to systemic host modifications exhibited by an accretion in acute phase reactants. These comprise infections by Gram-positive and Gram-negative bacteria. Despite the fact that the revelation of inflated elevation of CRP in the serum is not a clear-cut for each distinct condition, it is an appropriate sign of inflammatory pathway. As raised CRP values are frequently correlated to pathological alteration, the CRP evaluation contributes beneficial communication for the interpretation and pointing out of inflammatory cascade and related inflammatory disease.[10] Established facts drew from many studies[10,11] that sequential CRP assessment perhaps accepting as a diagnostic tool for clinical infections, deciding effects of treatment, after effect, and early disclosure of recurrence of the disease, and as a deduction may be a convenient distinguishing tool in deciding disease advancement. The complete capacity of inflamed periodontal tissue may also play a role, and there is an impulse for elevated CRP levels in generalized periodontitis correlated to localized periodontitis. Established fact is that the intensity of periodontal inflammation and the magnitude of bacterial spread are directly proportional to each other. Succeeding, the systemically scattered bacteria and lipopolysaccharide, besides cytokines from the periodontal lesion, may encourage hepatocytes and circulate leukocytes to generate CRP and interleukin-6, mutually.[11,12] Concluding remarks of the other studies that the periodontal treatment downturns the elevated CRP levels.[13]
CLM is an acclaimed macrolide, among a broad antimicrobial spectrum, competent contending on periodontopathic microorganisms. The main antibacterial action of this drug brings to bear the protein synthesis by binding to the 50S ribosomal subunit. Accomplishing higher levels of CLM in gingiva than serum and in the inflamed gingiva than the healthy gingiva were conclusively proven. Its distribution profile seems to be acceptable for therapy of periodontitis.[14]
Gram-negative microbial biofilm, which initiates the periodontal diseases, eventually turns out to osseous and soft-tissue destruction which leads to the pocket formation. Admitting that SRP is persuasive, it has assured limitations such as outright calculus eradication is impractical for teeth that have probing depth more than 4 mm, posterior teeth with furcation involvement, or in teeth with aberrant configuration. Thus, an accessory to SRP is inevitably focused at regulating the bacterial flora consolidated with the periodontium.[15]
Antimicrobials, which are given systemically, have corroborated to be constructive effects of nonsurgical and surgical approaches. A contemporary methodical analysis commences that suitable application of systemic antibiotics in periodontal chemotherapy; in concomitance with SRP undoubtedly improves the clinical and microbiological treatment outcome.[16] This study results also in accordance with the above study results, in this study also clinical parameters PD was significantly reduced at 3 months of the period, and microbiological parameters Aa and Pg were also reduced from baseline to 3 months of the period.
The effect of CLM on periodontopathic microorganisms was well discussed in some studies;[17,18] in particular, Aa, Pg, Prevotella spp., Fusobacterium nucleatum, and distinct added periodontopathic bacteria. The superior quality of this drug compared to other drugs is exceeding bioavailability, favorable tissue allocation, and a depressed occurrence of antagonistic after-effects.[19] This study results are also in accordance with these studies, Aa and Pg levels were reduced in 3 months of a period after administration of CLM.
Many in vitro studies[19,20] have shown that extensive intracellular concentrations of CLM represent the active transportation. Intracellular accumulation of CLM in gingival fibroblasts and oral epithelial cells is 38-fold higher than extracellular levels that are 3.3-fold only. Antecedent analogizes[21] have commended that it is agreeable to predict the CLM absorption to be prominent at inflamed sites than at healthy sites. 500 mg of CLM, three times daily for 7 days, was administered in this study. This quantity was expressed to be efficacious to periodontal tissue by the foregoing analysis.[18] When we compared this study results from baseline to 3 and 6 months, at the 3 months period, a significant reduction was seen; and at the 6 months period, gradually the levels were increased. The fluctuating values from disease to health and health to disease were fulfilling the above statement. This also might be one of the reasons for shorter action of the drug.
“Aa” is a Gram-negative microaerophilic coccobacillus that is firmly leagued with early-onset periodontitis and refractory adult periodontitis. Pg is a Gram-negative anaerobic rod that is combined with severe adult periodontitis. Both possess virulence factors that frustrate the host response.[22] “Aa” is a challenging therapeutic target that invades epithelial cells, where they can multiply and spread into adjacent tissues. Recent studies have found high levels of “Aa” inside epithelial cells associated with periodontal pockets, gingival crevices, and buccal mucosa in healthy individuals and patients with chronic periodontitis. “Aa” releases a leukotoxin that forms pores in the plasma membrane of polymorphonuclear (PMNs) leukocytes, causing osmotic instability, and cell death. It is also capable of inhibiting PMN chemotaxis and impairing production of antibacterial agents such as hydrogen peroxide. For these reasons, it is difficult for PMNs to eradicate this pathogen. Resultantly, antibiotic adjunct such as CLM conceivably required.[23] Some authors Piccolomini et al.[24] and Iskandar et al.[25] concluded that CLM is effective against “Aa” and “Pg” human leukocyte-60 cells and mature human PMNs both possess an active transporter that takes up CLM. It is well suited for accumulating high intracellular concentrations of this agent under conditions found in the inflamed gingiva.[23,24,25]
Some authors’ tried CLM as local drug delivery as well as systemic tablet form concluded that statistically significant reduction in periodontopathic organisms from baseline to 9-month intervals.[26,27] Despite the fact that, SRP is a pragmatic approach for fundamental treatment of chronic periodontitis; on the other hand, it fails to exterminate subgingival pathogens and standstill, the accelerating attachment loss in some patients. Clinical attachment gain and probing depth devaluation will be seen usually subsequential achievement of the SRP, these actions might embellish by the ancillary administration of antibiotics right away after attainment of the nonsurgical periodontal therapy.[28,29,30]
Limitations of this study as follows: CLM administration was given for only 7 days, committed on preceding descriptions, and extended systematic plan may have claimed greater benefits. Furthermore, the sample size in this investigation was limited; anyway, reinforced research with considerable sample size is required to validate the efficacy of this macrolide. Moreover, the effect of CLM was not evaluated for an exact period.
CONCLUSIONS
Systemic antibiotic therapy executes the subgingival pathogens that survive after periodontal instrumentation. Although CLM has not been widely used in periodontal therapy, it was shown to be effective against “Aa” and several other periodontal pathogens, as it can reach bacteria that have invaded oral epithelium.[31] In combination with results from previous studies,[32,33,34] the current findings suggest that CLM could be useful for treating periodontitis. However, an affirmative effect of CLM is very narrow. The use of this antibiotic in the countermeasure of patients with periodontitis is exclusive for a limited period. As per this study results, it was effective only within 3 months of period. Different interventional studies related to dose variation and longer duration of drug usage might elucidate the dissimilar results. To get the accurate results, further long-run studies related to tissue invasion and intercellular accumulation of CLM with advanced diagnostic methods such as polymerase chain reaction and immunohistochemistry analysis might attain favorable results.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The authors would like to acknowledge Alice Rani, Dr. Sukesh Kumar for samples culturing and processing and also to Dr. John Manoz and Doshi Dolar for editing.
REFERENCES
- 1.Jepsen K, Jepsen S. Antibiotics/antimicrobials: Systemic and local administration in the therapy of mild to moderately advanced periodontitis. Periodontol 2000. 2016;71:82–112. doi: 10.1111/prd.12121. [DOI] [PubMed] [Google Scholar]
- 2.Marsh PD. Dental plaque: Biological significance of a biofilm and community life-style. J Clin Periodontol. 2005;32(Suppl 6):7–15. doi: 10.1111/j.1600-051X.2005.00790.x. [DOI] [PubMed] [Google Scholar]
- 3.Zhou J, Yao Y, Jiao K, Zhang J, Zheng X, Wu F, et al. Relationship between gingival crevicular fluid microbiota and cytokine profile in periodontal host homeostasis. Front Microbiol. 2017;8:2144. doi: 10.3389/fmicb.2017.02144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iskandar I, Walters JD. Clarithromycin accumulation by phagocytes and its effect on killing of Aggregatibacter actinomycetemcomitans. J Periodontol. 2011;82:497–504. doi: 10.1902/jop.2010.100221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.López R, Baelum V. Periodontal disease classifications revisited. Eur J Oral Sci. 2015;123:385–9. doi: 10.1111/eos.12227. [DOI] [PubMed] [Google Scholar]
- 6.Marya CM. New Delhi, St. Louis, Panama, London: Jaypee Brother's Medical Publishers (P) Ltd; 2011. Dental indices. A Textbook of Public Health Dentistry; pp. 185–212. [Google Scholar]
- 7.Herrera D, Sanz M, Jepsen S, Needleman I, Roldán S. A systematic review on the effect of systemic antimicrobials as an adjunct to scaling and root planing in periodontitis patients. J Clin Periodontol. 2002;29(Suppl 3):136–59. doi: 10.1034/j.1600-051x.29.s3.8.x. [DOI] [PubMed] [Google Scholar]
- 8.Haffajee AD, Socransky SS, Gunsolley JC. Systemic anti-infective periodontal therapy. A systematic review. Ann Periodontol. 2003;8:115–81. doi: 10.1902/annals.2003.8.1.115. [DOI] [PubMed] [Google Scholar]
- 9.Garcia Canas P, Khouly I, Sanz J, Loomer PM. Effectiveness of systemic antimicrobial therapy in combination with scaling and root planing in the treatment of periodontitis: A systematic review. J Am Dent Assoc. 2015;146:150–63. doi: 10.1016/j.adaj.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 10.Bansal T, Pandey A, Deepa D, Asthana AK. C-reactive protein (CRP) and its association with periodontal disease: A brief review. J Clin Diagn Res. 2014;8:ZE21–4. doi: 10.7860/JCDR/2014/8355.4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amar S, Han X. The impact of periodontal infection on systemic diseases. Med Sci Monit. 2003;9:RA291–9. [PubMed] [Google Scholar]
- 12.Ramamoorthy RD, Nallasamy V, Reddy R, Esther N, Maruthappan Y. A review of C-reactive protein: A diagnostic indicator in periodontal medicine. J Pharm Bioallied Sci. 2012;4:S422–6. doi: 10.4103/0975-7406.100318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yazdi FK, Karimi N, Rasouli M, Roozbeh J. Effect of nonsurgical periodontal treatment on C-reactive protein levels in maintenance hemodialysis patients. Ren Fail. 2013;35:711–7. doi: 10.3109/0886022X.2013.777890. [DOI] [PubMed] [Google Scholar]
- 14.Burrell RC, Walters JD. Distribution of systemic clarithromycin to gingiva. J Periodontol. 2008;79:1712–8. doi: 10.1902/jop.2008.080013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kolakovic M, Held U, Schmidlin PR, Sahrmann P. An estimate of pocket closure and avoided needs of surgery after scaling and root planing with systemic antibiotics: A systematic review. BMC Oral Health. 2014;14:159. doi: 10.1186/1472-6831-14-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Albandar JM. Adjunctive antibiotics with nonsurgical periodontal therapy improve the clinical outcome of chronic periodontitis in current smokers. J Evid Based Dent Pract. 2012;12:63–6. doi: 10.1016/S1532-3382(12)70015-5. [DOI] [PubMed] [Google Scholar]
- 17.Andere NM, Castro Dos Santos NC, Araujo CF, Mathias IF, Taiete T, Casarin RC, et al. Clarithromycin as an adjunct to one-stage full-mouth ultrasonic periodontal debridement in generalized aggressive periodontitis: A randomized controlled clinical trial. J Periodontol. 2017;88:1244–52. doi: 10.1902/jop.2017.170165. [DOI] [PubMed] [Google Scholar]
- 18.Pradeep AR, Kathariya R. Clarithromycin, as an adjunct to non surgical periodontal therapy for chronic periodontitis: A double blinded, placebo controlled, randomized clinical trial. Arch Oral Biol. 2011;56:1112–9. doi: 10.1016/j.archoralbio.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 19.Soares GM, Figueiredo LC, Faveri M, Cortelli SC, Duarte PM, Feres M. Mechanisms of action of systemic antibiotics used in periodontal treatment and mechanisms of bacterial resistance to these drugs. J Appl Oral Sci. 2012;20:295–309. doi: 10.1590/S1678-77572012000300002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Bambeke F, Barcia-Macay M, Lemaire S, Tulkens PM. Cellular pharmacodynamics and pharmacokinetics of antibiotics: Current views and perspectives. Curr Opin Drug Discov Devel. 2006;9:218–30. [PubMed] [Google Scholar]
- 21.McConnell SA, Amsden GW. Review and comparison of advanced-generation macrolides clarithromycin and dirithromycin. Pharmacotherapy. 1999;19:404–15. doi: 10.1592/phco.19.6.404.31054. [DOI] [PubMed] [Google Scholar]
- 22.Torrungruang K, Jitpakdeebordin S, Charatkulangkun O, Gleebbua Y. Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitans, and Treponema denticola/Prevotella intermedia co-infection are associated with severe periodontitis in a Thai population. PLoS One. 2015;10:e0136646. doi: 10.1371/journal.pone.0136646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Konstantinidis T, Kambas K, Mitsios A, Panopoulou M, Tsironidou V, Dellaporta E, et al. Immunomodulatory role of clarithromycin in Acinetobacter baumannii infection via formation of neutrophil extracellular traps. Antimicrob Agents Chemother. 2016;60:1040–8. doi: 10.1128/AAC.02063-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piccolomini R, Catamo G, Di Bonaventura G. Bacteriostatic and bactericidal in vitro activities of clarithromycin and erythromycin against periodontopathic Actinobacillus actinomycetemcomitans. Antimicrob Agents Chemother. 1998;42:3000–1. doi: 10.1128/aac.42.11.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raghunatha K, George JP. Periodontal tissue and serum concentration of clarithromycin after systemic administration in patients affected by chronic periodontitis. J Periodontol. 2013;84:e17–22. doi: 10.1902/jop.2013.120521. [DOI] [PubMed] [Google Scholar]
- 26.Agarwal E, Pradeep AR, Bajaj P, Naik SB. Efficacy of local drug delivery of 0.5% clarithromycin gel as an adjunct to non-surgical periodontal therapy in the treatment of current smokers with chronic periodontitis: A randomized controlled clinical trial. J Periodontol. 2012;83:1155–63. doi: 10.1902/jop.2012.110600. [DOI] [PubMed] [Google Scholar]
- 27.Kathariya R, Pradeep AR, Raghavendra NM, Gaikwad R. Evaluation of subgingivally delivered 0.5% clarithromycin as an adjunct to nonsurgical mechanotherapy in the management of chronic periodontitis: A short-term double blinded randomized control trial. J Investig Clin Dent. 2014;5:23–31. doi: 10.1111/jicd.12009. [DOI] [PubMed] [Google Scholar]
- 28.Bragd L, Dahlén G, Wikström M, Slots J. The capability of Actinobacillus actinomycetemcomitans, Bacteroides gingivalis and Bacteroides intermedius to indicate progressive periodontitis; a retrospective study. J Clin Periodontol. 1987;14:95–9. doi: 10.1111/j.1600-051x.1987.tb00949.x. [DOI] [PubMed] [Google Scholar]
- 29.Chou CH, Walters JD. Clarithromycin transport by gingival fibroblasts and epithelial cells. J Dent Res. 2008;87:777–81. doi: 10.1177/154405910808700812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slots J. Selection of antimicrobial agents in periodontal therapy. J Periodontal Res. 2002;37:389–98. doi: 10.1034/j.1600-0765.2002.00004.x. [DOI] [PubMed] [Google Scholar]
- 31.Tribble GD, Lamont RJ. Bacterial invasion of epithelial cells and spreading in periodontal tissue. Periodontol 2000. 2010;52:68–83. doi: 10.1111/j.1600-0757.2009.00323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bollen CM, Quirynen M. Microbiological response to mechanical treatment in combination with adjunctive therapy. A review of the literature. J Periodontol. 1996;67:1143–58. doi: 10.1902/jop.1996.67.11.1143. [DOI] [PubMed] [Google Scholar]
- 33.Walters J, Lai PC. Should antibiotics be prescribed to treat chronic periodontitis? Dent Clin North Am. 2015;59:919–33. doi: 10.1016/j.cden.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ye P, Chang J, Foo LF, Yap BC. An early report: A modified porphyrin-linked metronidazole targeting intracellular Porphyromonas gingivalis in cultured oral epithelial cells. Int J Oral Sci. 2017;9:167–73. doi: 10.1038/ijos.2017.31. [DOI] [PMC free article] [PubMed] [Google Scholar]


