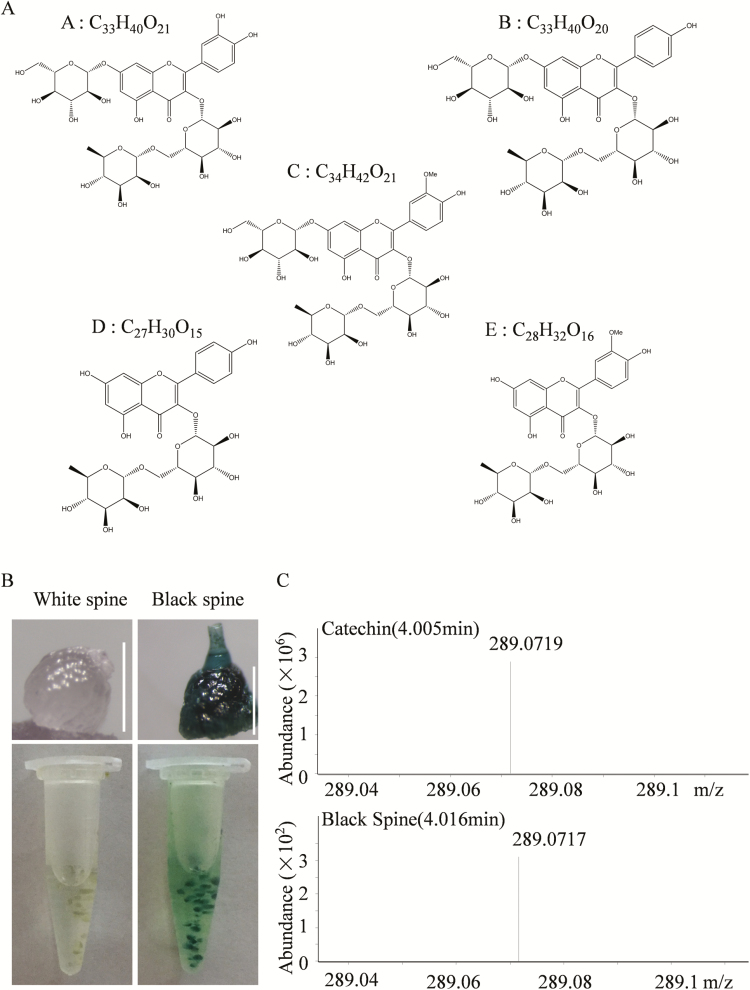
Fig. 4.
Molecular formulae and structures of the five flavonols identified by NMR, and identification of PAs in black spines of line RNS9. (A) The formulae and structures of A, quercetin-3-O-rutinoside -7-O-glucose; B, kempferol 3-O-rutinoside 7-O-glucoside; C, isorhamnetin -3-O-rutinoside-7-O-glucoside; D, kaempferol -3-O- rutinoside; and E, isorhamnetin- 3-O-rutinoside. The NMR physicochemical and spectral data are listed in Supplementary Fig. S5. (B) DMACA (p-dimethylaminocinnamaldehyde) staining of white and black spines (white spines of the RNS8 line). The images show single stained spines (top) and many stained spines (bottom). Scale bars are 1 mm. (C) Confirmation of proanthocyanidins in black spines as determined by tandem MS using catechin as a control. The MS ion peak signal for the catechin monomer is shown (m/z 289.0718 ± 5 ppm).