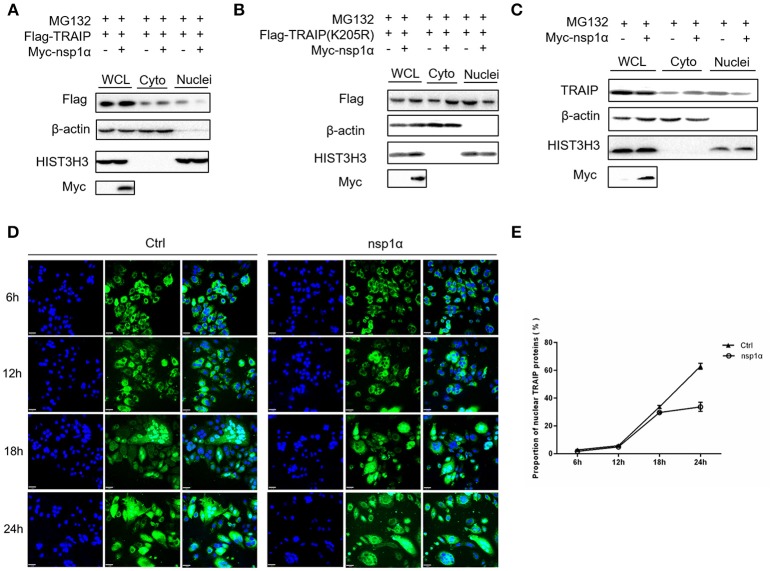
Figure 8.
Nsp1α decreased the abundance of TRAIP in the nucleus. HEK 293T cells were co-transfected with Myc-nsp1α and Flag-TRAIP (WT) (A) or Flag-TRAIP (K205R) (B), in the presence of proteasome inhibitor MG132 (5 μM) and after 24 h, cytoplasmic protein and nucleoprotein were extracted and detected with anti-Flag mAb or anti-Myc antibody, respectively, by Western blotting. HIST3H3 was used as an internal loading control for nuclear protein load, and β-actin as the control for cytoplasmic protein load. (C) HEK 293T cells were transfected with Myc-nsp1α. At 24 h post-transfection, cytoplasmic protein and nucleoprotein were extracted and detected with TRAIP mAb or anti-Myc antibody. (D) Co-transfection of Flag-TRAIP with Myc-nsp1α or vector into HeLa cells. The cells were fixed and double-stained with a mouse anti-Flag antibody at the indicated times, followed by FITC-conjugated anti-mouse IgG (green). Nuclei were stained with DAPI (blue). Cells were observed under a laser confocal imaging analysis system, scale bar: 7 μm. (E) Statistical analysis of TRAIP distribution in cytoplasm and nucleus in HeLa cells was counted.