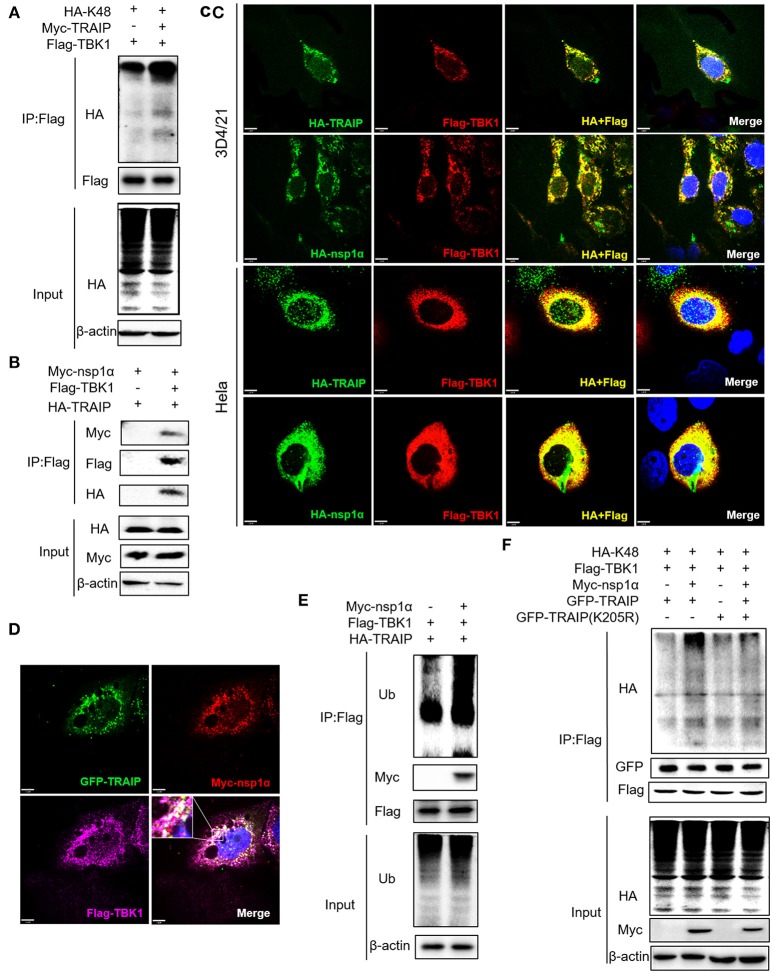
Figure 9.
Nsp1α forms trimer complex with TRAIP and TBK1. (A,F) Detection of Lys48 (K48)-linked polyubiquitination of TBK1. HEK 293T cells were transfected with Flag-TBK1, Myc-TRAIP, HA-K48, or Myc-empty vector (A) or HA-K48, GFP-TRAIP or GFP-TRAIP (K205R), Flag-TBK1, Myc-nsp1α, or Myc-empty vector (F). At 24 h post-transfection, FLAG-immunoprecipitates were probed with HA antibodies to detect to Lys48 (K48)-linked polyubiquitination of TBK1. (B) HEK293T cells were co-transfected with Myc-nsp1α, HA-TRAIP, Flag-TBK1, or Flag-empty vector. FLAG immunoprecipitation (IP) was used to detect the interaction of TBK1, nsp1α, and TRAIP. (C) Immunofluorescence was used to detect the interaction between TBK1, nsp1α, and TRAIP in 3D4/21 and HeLa cells. (D) Co-transfection of GFP-TRAIP with Myc-nsp1α and Flag-TBK1 into HeLa cells. The cells were fixed and double-stained with a mouse anti-Myc antibody and a rabbit anti-Flag antibody and followed by PE-conjugated anti-mouse IgG (red) and IF647 goat anti-rabbit IgG. Nuclei were stained with DAPI (blue). Cells were observed under a laser confocal imaging analysis system, scale bar: 7 μm. (E) HEK 293T cells were transfected with Flag-TBK1, HA-TRAIP, or Myc-empty vector. At 24 h post-transfection, FLAG-immunoprecipitates were probed with Ub antibodies to detect polyubiquitination of TBK1.