Abstract
Various therapeutic modalities including radiofrequency ablation, cryoablation, microwave ablation, and irreversible electroporation have attracted attention as energy sources for effective locoregional treatment of hepatocellular carcinoma (HCC); these are accepted non-surgical treatments that provide excellent local tumor control and favorable survival. However, in contrast to surgery, tumor location is a crucial factor in the outcomes of locoregional treatment because such treatment is mainly performed using a percutaneous approach for minimal invasiveness; accordingly, it has a limited range of ablation volume. When the index tumor is near large blood vessels, the blood flow drags thermal energy away from the targeted tissue, resulting in reduced ablation volume through a so-called “heat-sink effect”. This modifies the size and shape of the ablation zone considerably. In addition, serious complications including infarction or aggressive tumor recurrence can be observed during follow-up after ablation for perivascular tumors by mechanical or thermal damage. Therefore, perivascular locations of HCC adjacent to large intrahepatic vessels can affect post-treatment outcomes. In this review, we primarily focus on physical properties of perivascular tumor location, characteristics of perivascular HCC, potential complications, and clinical outcomes after various locoregional treatments; moreover, we discuss the current status and future perspectives regarding percutaneous ablation for perivascular HCC.
Keywords: Hepatocellular carcinoma, Perivascular, Radiofrequency ablation, Liver, Cryoablation, Microwave ablation, Irreversible electroporation
Core tip: Recently safety concerns have been raised regarding the risks of radiofrequency (RF) ablation for perivascular hepatocellular carcinomas (HCCs), due to the risks of ischemic complications and intravascular tumor spread during treatment. To overcome these potential risks, a modified RF ablation technique, cryoablation, combined treatment with transarterial chemoembolization, or microwave ablation could be problem-solving tools for the treatment of perivascular HCCs. However, the effectiveness of these techniques should be validated with further prospective studies due to the lack of current evidence.
INTRODUCTION
Image-guided tumor ablation is an evolving and growing treatment option for patients with hepatocellular carcinoma (HCC). This local treatment offers significant advantages, as it is less invasive than surgery and demonstrates a low risk of major complications[1]. In contrast to surgical resection with a laparoscopic or open approach, tumor location is a crucial factor in the outcomes of local ablation therapy, because it is primarily performed by using a percutaneous approach for minimal invasiveness[2].
Although ablation technology has evolved and grown rapidly during the past decades, such that it can help improve clinical outcomes and safety profiles[3], high-risk locations of HCC adjacent to extrahepatic vital organs or large intrahepatic vessels exhibit increased risks of complications after local ablation therapy[4]. In particular, there remain controversies regarding the outcomes of local treatment because of the heat-sink effect, which can considerably modify the size of the ablation zone, in patients with perivascular tumors[5,6]. In addition, perivascular HCC exhibits different underlying tumor characteristics, which can have profound effects on the resulting poor prognosis after local ablation therapy[7]. A previous study also suggested that the iatrogenic transportal tumor spread may occur during radiofrequency (RF) ablation for periportal tumors[8] because these local therapies cannot remove a hepatic segment confined to tumor-bearing portal tributaries, unlike anatomical surgical resection.
For improvement of patient outcomes with respect to perivascular HCCs, a modified RF ablation technique and other new energy sources for ablation therapies have recently been introduced. An understanding of these new aspects is important for optimizing clinical results. In particular, combining an understanding of the specific characteristics of each ablation modality with an understanding of the characteristics of perivascular HCC, and then selecting the most appropriate ablation modality available for each patient, can have a remarkable effect on patient outcomes. This review can help physicians to plan state-of-the-art local ablation treatment for patients with perivascular HCC.
Definition of perivascular HCC
To date, there is no universal consensus definition regarding perivascular HCC. The optimal threshold of the contacting vessel size has been based on the results of an experimental study that used pigs[9]. Notably, most veins greater than 3 mm remained patent after RF ablation; there was an invagination of residual viable tissue between vessels and the RF ablation zone, known as the “heat-sink effect”. Many subsequent clinical studies[5-7,10,11] adopted corresponding definitions of perivascular tumor; an index tumor was characterized by any contact with first or second degree branches of a portal or hepatic vein that are 3 mm or greater in diameter.
Specific ablation environment in perivascular HCC
Unlike en bloc tissue removal by surgical resection, RF or microwave ablation uses thermal energy from the RF electric current or microwave field to destroy cancer cells[12]. However, when the index tumor is near large blood vessels, the blood flow carries thermal energy away from the targeted tissue, resulting in reduced ablation volume; this considerably modifies the size and shape of the ablation zone, especially during RF ablation[9]. Similarly, the same phenomenon can happen during cryoablation. The convective influx of circulating warm blood into a frozen tumor would theoretically make the ablation of perivascular tumor tissue insufficient[13].
Specific tumor environment for perivascular HCC
According to recent Barcelona Clinic Liver Cancer guidelines[1], macroscopic vascular invasion into the portal or hepatic vein is a key factor for staging in patients with HCC due to poor prognosis, despite curative treatment. In addition, microvascular invasion of HCC, which cannot be easily diagnosed by preoperative imaging studies, is another important indicator of poor prognosis after surgical resection[14] and liver transplantation[15]. Thus, perivascular tumors are more likely to be exposed to these substantial risks of vascular invasion, compared with non-perivascular tumors; this difference may lead to poor patient outcomes. Although some researchers[16] have shown that post-operative adjuvant transarterial chemoembolization (TACE) after surgical resection improved outcomes among patients who exhibit HCC with microvascular invasion, there remains uncertainty with respect to adjuvant therapy after curative treatment for HCC, with either micro- or macro-vascular invasion because a potent anticancer drug for HCC is not well established in clinical practice.
RF ablation
The mechanism of RF ablation uses electric current to rapidly oscillate tissue ions, creating frictional heating in areas of high current density adjacent to the electrode[12]. Thus, growth of the ablation zone primarily depends on thermal diffusion; this process could be limited by the “heat-sink effect” from peritumoral vessels. Previous studies[5,11] showed a significant correlation between the presence of peritumoral vessel and poor local tumor control during RF ablation for HCCs. Among these investigations, Lu et al[11] reported that the presence of a peritumoral vessel is a significant factor for incomplete treatment in RF ablation (53% in perivascular HCC vs 12% in non-perivascular HCC), as verified during histologic examination of explanted liver after transplantation. In contrast to the aforementioned studies, several other studies[6,10,17,18] investigating the same topic reported similar therapeutic outcomes between perivascular and non-perivascular HCCs; the presence of a peritumoral vessel was not an independent factor associated with incomplete tumor ablation. Improvements in the outcomes of more recent studies of RF ablation for perivascular HCC may be attributed to advances in technical factors, including RF ablation strategies such as the no-touch technique, the use of multiple or large electrodes, a more powerful generator, or advancements in power deploy algorithms[19].
Regarding clinical outcomes associated with specific types of peritumoral vessels in RF ablation, Lee et al[7] demonstrated a significant interaction effect between RF ablation and type of peritumoral vessel, with respect to extrahepatic recurrence or overall survival. Although this study did not reveal the cause of different outcomes according to the type of peritumoral vessel, these results could support an increased risk of extrahepatic recurrence when performing RF ablation for periportal HCCs, compared with RF ablation for perivenous HCCs; this increased risk may affect survival outcome. The hemodynamics of blood flow differ considerably between the two types of hepatic vessels[20]; this could lead to a different ablation environment when performing RF ablation for HCC. Therefore, future studies should consider the type of peritumoral vessel during assessment of the outcomes of RF ablation in patients with perivascular HCCs.
With respect to tumor location, Kang et al[8] reported that periportal tumor location was a risk factor for aggressive intrasegmental recurrence after RF ablation (Figure 1). Although the exact mechanism of this type of tumor recurrence remains unclear, intravascular tumor spread along the peritumoral portal vein may be a primary cause of such complications. During insertion of the RF electrode, abnormal communication of an iatrogenic arterioportal fistula may develop; this may enable cancer cells to spread into the peripheral liver due to ablation-related mechanical injury[21]. In addition, rapid heating of a HCC can lead to a sudden increase in the internal pressure of ablated tissue, which may cause unintentional scattering of tumor cells around the ablation zone[22,23]. To prevent this potential vascular complication, potential approaches include the no-touch multi-polar ablation technique without direct puncture of the index tumor[24,25], longer ablation times with stepwise power increment at lower power[23], combined RF ablation treatments with TACE[26], or cryoablation[13]; these problem solving tools may be especially effective in patients with periportal HCCs. The effectiveness of these techniques should be validated with further prospective studies.
Figure 1.
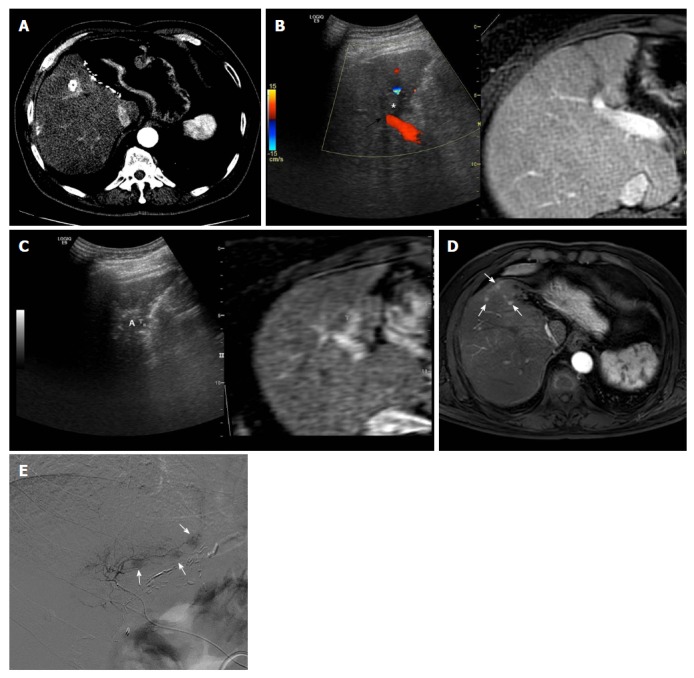
Images demonstrating aggressive intrasegmental recurrence after radiofrequency ablation for perivascular hepatocellular carcinoma. A: Axial computed tomography image obtained during hepatic arterial phase shows viable hepatocellular carcinoma (HCC) within the partially lipiodolized nodule (asterisk) in segment V before radiofrequency (RF) ablation. The index tumor is in contact with the right portal vein (black arrow); B: On planning ultrasonography (US), using fusion imaging with color Doppler US and magnetic resonance imaging (MRI), the low echogenic incident tumor (asterisk) is in contact with a right portal vein (black arrow); C: During RF ablation with the US fusion system, the ablation zone (A) is covered with viable enhancing tumor foci, indicating T marker on real time US/fused MR image; D: MRI scan obtained during the hepatic arterial phase 9 mo after RF ablation shows multiple small arterial enhancing nodules (white arrows) of consistent size, representing recurrent tumors. These recurrent tumors developed simultaneously in a peripheral area of the treated segment, fed by the previous peritumoral portal vein; E: The patient underwent transarterial chemoembolization for tumor control considering tumor multiplicity. Multiple small nodular tumors were detected along the portal tract on hepatic angiogram.
Cryoablation
Despite the absence of thermal injury and superb visualization of the procedure process, cryoablation for HCC has been used much less frequently than RF ablation. This is because large cryoprobes with bulky liquid nitrogen systems under laparotomy setting were used in the early era of cryoablation[27]. Thus, although serious complications were rare, excessive bleeding and cryoshock related to the procedure were reported[28]. However, a new generation of cryoablation systems with thin cryoprobes that use argon-helium has been introduced[29] and recent randomized controlled trials showed that they were equally safe and effective compared with RF ablation[30]. Cryoablation systems use the Joule-Thomson theory of expanding gases within a needlelike cryoprobe[12]. The mechanism of cell death with ice-ball formation involves cell membrane disruption and an associated release of intracellular contents[31]. Unlike RF ablation, cryoablation for perivascular HCC could show a better safety profile with respect to vascular complications, such as hepatic infarction or peritumoral vessel thrombosis, because the ablation zone is rapidly reperfused after the ice ball has melted. A previous study[6] regarding hepatic infarction after RF ablation reported an incidence of 5% in patients with HCC, due to the frequent development of thrombosis in peritumoral vessels by thermal injury (Figure 2). However, Kim et al[13] reported that persistent thrombosis of peritumoral vessels was 3.4%; no case of hepatic infarction was observed in patients who underwent cryoablation for perivascular HCCs. In clinical practice, local ablation therapy is preferred when the patient exhibits recurrent tumors after surgical resection, because more limited hepatic functional reserve is expected[32]. In these particular scenarios, cryoablation may be the most effective local ablation modality in patients with limited hepatic reserve due to the very low risk of procedure-related vascular complications, including hepatic infarction. In addition, to the best of our knowledge, there have been no reports regarding aggressive tumor recurrence after cryoablation for perivascular HCC. Based on the absence of thermal expansion of ablation zones, as is observed in RF or microwave ablation, cryoablation theoretically might constitute a safer ablation method with respect to the possibility of tumor spread through an increase in the internal pressure of ablated tissue. However, insufficient data are available and further studies are required to validate the long-term safety of cryoablation for perivascular HCCs.
Figure 2.
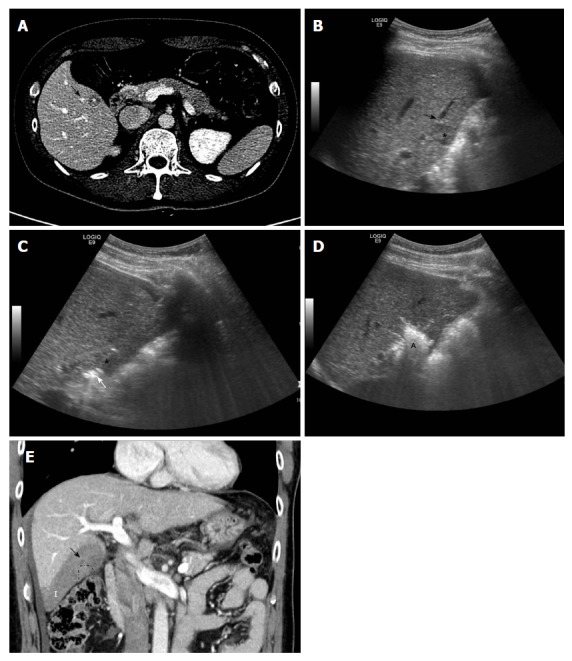
Images showing subsegmental hepatic infarction after radiofrequency ablation for perivascular hepatocellular carcinoma. A: Axial computed tomography image obtained during equilibrium phase shows 1.3-cm hepatocellular carcinoma (asterisk) in segment V before radiofrequency (RF) ablation. The index tumor is in contact with the right portal vein (black arrow); B: Planning ultrasound image obtained before RF ablation shows the low-echoic-index tumor (asterisk) in contact with a right portal vein (black arrow); C: During RF ablation, the RF electrode (white arrow) is inserted into the index tumor (asterisk), evading the adjacent portal vein; D: At the end of the procedure, a hyperechoic ablation zone (A) completely covered the index tumor; E: Thrombosis within the peritumoral portal vein (black arrow) developed around the index tumor (dotted line), shown on coronal computed tomography images obtained immediately after RF ablation. This led to subsegmental infarction (I) in the peripheral area of hepatic segment VI.
Microwave ablation
Although previous first- or second-generation microwave ablation system was limited due to lack of active antenna cooling and low power generator, recent third-generation systems incorporates antenna cooling and high power generators[12]. In microwave heating, polar molecules continuously realign with the oscillating microwave field, effectively increasing kinetic energy and tissue temperature[12]. As a result, microwave energy may possess several advantages, compared with RF ablation; these include faster heating with a larger ablation volume, higher intratumoral temperatures, and less dependence on the electrical conductivities of tissue[33]. These characteristics of microwave ablation may render it less affected by the “heat-sink effect” present in perivascular tissue[34]. In addition, combined TACE and microwave ablation could increase local tumor control for perivascular tumor resulting from the complementary nature of the two different treatments[35]. To be specific, TACE can decrease blood flow and thereby decrease perfusion-mediated cooling, allowing the creation of larger ablation zones even in perivascular tumor location[36]. Furthermore, there could be substantial synergistic effect of applying thermal ablation to a chemotherapeutic agent-laden tumor[30]. Recent report showed that local tumor control, overall survival, and major complications in the perivascular and non-perivascular groups are not significantly different when performing microwave ablation for HCCs. However, there has been no study directly comparing RF ablation and microwave ablation for perivascular HCCs. In addition, whether the ability of microwave ablation to induce a broader ablation zone can lead to a real survival benefit remains unclear. Although randomized controlled trials are difficult to perform in such a rapidly evolving field, additional trials are required to confirm these debates.
Irreversible electroporation
Irreversible electroporation (IRE) is a novel, non-thermal form of tumor ablation that uses high-current electrical pulses to induce pore formation in the cell lipid bilayer, resulting in cell death[37]. Thus, it is not affected by the “heat-sink effect” and may cause less collateral damage based on its mechanism of action. Through several studies investigating IRE for hepatic tumors[38-40], IRE has been shown to be safe and acceptable for local tumor control, especially with regard to use within close proximity to the venous systems of the liver; this is a notable advantage for IRE. However, given the paucity of long-term data demonstrating safety and efficacy for the treatment of HCC, IRE largely serves as a niche technology for the ablation of small (< 3 cm), unresectable tumors, which are not amenable to thermal ablation due to the abutment of major vessels or hilar structures[41].
CONCLUSION
Image-guided tumor ablation is becoming increasingly accepted for the treatment of very early and early stage HCC. Ablative treatments, particularly RF ablation, currently represent the first-line option for patients with unresectable early-stage HCC. However, safety concerns have been raised regarding the risks of RF ablation for perivascular HCCs, due to the risks of ischemic complications and intravascular tumor spread during treatment. To overcome these potential risks, a modified RF ablation technique, cryoablation, microwave ablation, or combined treatment with TACE have been used recently. Especially, microwave ablation has potential physical advantages over RF ablation and it may be beneficial in treating perivascular tumors. However, additional prospective studies are needed to assess whether the recent technical advances of RF ablation and ablation therapies with new energy sources can translate into better clinical outcomes for patients with perivascular HCC, compared with conventional RF ablation. We hope that understanding the characteristics of perivascular tumor locations and the current status of each ablation modality could help overcome difficulties related to the treatment of perivascular HCCs, and ultimately provide meaningful improvements in patient outcomes.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
Conflict-of-interest statement: The authors do not have any conflicts of interest to declare.
Peer-review started: September 19, 2018
First decision: October 16, 2018
Article in press: November 2, 2018
P- Reviewer: Iannitti DA, Sartori S, Shousha HI S- Editor: Ma RY L- Editor: A E- Editor: Huang Y
Contributor Information
Tae Wook Kang, Department of Radiology and Center for Imaging Science, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul 135-710, South Korea.
Hyo Keun Lim, Department of Radiology and Center for Imaging Science, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul 135-710, South Korea; Department of Health Sciences and Technology, SAIHST, Sungkyunkwan University, Seoul 135-710, South Korea. rfalim@skku.edu.
Dong Ik Cha, Department of Radiology and Center for Imaging Science, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul 135-710, South Korea.
References
- 1.European Association for the Study of the Liver. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 2.Mulier S, Ni Y, Jamart J, Ruers T, Marchal G, Michel L. Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann Surg. 2005;242:158–171. doi: 10.1097/01.sla.0000171032.99149.fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kang TW, Rhim H. Recent Advances in Tumor Ablation for Hepatocellular Carcinoma. Liver Cancer. 2015;4:176–187. doi: 10.1159/000367740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teratani T, Yoshida H, Shiina S, Obi S, Sato S, Tateishi R, Mine N, Kondo Y, Kawabe T, Omata M. Radiofrequency ablation for hepatocellular carcinoma in so-called high-risk locations. Hepatology. 2006;43:1101–1108. doi: 10.1002/hep.21164. [DOI] [PubMed] [Google Scholar]
- 5.Lu DS, Raman SS, Limanond P, Aziz D, Economou J, Busuttil R, Sayre J. Influence of large peritumoral vessels on outcome of radiofrequency ablation of liver tumors. J Vasc Interv Radiol. 2003;14:1267–1274. doi: 10.1097/01.rvi.0000092666.72261.6b. [DOI] [PubMed] [Google Scholar]
- 6.Kang TW, Lim HK, Lee MW, Kim YS, Choi D, Rhim H. Perivascular versus nonperivascular small HCC treated with percutaneous RF ablation: retrospective comparison of long-term therapeutic outcomes. Radiology. 2014;270:888–899. doi: 10.1148/radiol.13130753. [DOI] [PubMed] [Google Scholar]
- 7.Lee S, Kang TW, Cha DI, Song KD, Lee MW, Rhim H, Lim HK, Sinn DH, Kim JM, Kim K. Radiofrequency ablation vs. surgery for perivascular hepatocellular carcinoma: Propensity score analyses of long-term outcomes. J Hepatol. 2018;69:70–78. doi: 10.1016/j.jhep.2018.02.026. [DOI] [PubMed] [Google Scholar]
- 8.Kang TW, Lim HK, Lee MW, Kim YS, Rhim H, Lee WJ, Gwak GY, Paik YH, Lim HY, Kim MJ. Aggressive Intrasegmental Recurrence of Hepatocellular Carcinoma after Radiofrequency Ablation: Risk Factors and Clinical Significance. Radiology. 2015;276:274–285. doi: 10.1148/radiol.15141215. [DOI] [PubMed] [Google Scholar]
- 9.Lu DS, Raman SS, Vodopich DJ, Wang M, Sayre J, Lassman C. Effect of vessel size on creation of hepatic radiofrequency lesions in pigs: assessment of the “heat sink” effect. AJR Am J Roentgenol. 2002;178:47–51. doi: 10.2214/ajr.178.1.1780047. [DOI] [PubMed] [Google Scholar]
- 10.Ng KK, Poon RT, Lam CM, Yuen J, Tso WK, Fan ST. Efficacy and safety of radiofrequency ablation for perivascular hepatocellular carcinoma without hepatic inflow occlusion. Br J Surg. 2006;93:440–447. doi: 10.1002/bjs.5267. [DOI] [PubMed] [Google Scholar]
- 11.Lu DS, Yu NC, Raman SS, Limanond P, Lassman C, Murray K, Tong MJ, Amado RG, Busuttil RW. Radiofrequency ablation of hepatocellular carcinoma: treatment success as defined by histologic examination of the explanted liver. Radiology. 2005;234:954–960. doi: 10.1148/radiol.2343040153. [DOI] [PubMed] [Google Scholar]
- 12.Hinshaw JL, Lubner MG, Ziemlewicz TJ, Lee FT Jr, Brace CL. Percutaneous tumor ablation tools: microwave, radiofrequency, or cryoablation--what should you use and why? Radiographics. 2014;34:1344–1362. doi: 10.1148/rg.345140054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim R, Kang TW, Cha DI, Song KD, Lee MW, Rhim H, Lim HK, Sinn DH. Percutaneous cryoablation for perivascular hepatocellular carcinoma: Therapeutic efficacy and vascular complications. Eur Radiol. 2018 doi: 10.1007/s00330-018-5617-6. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 14.Sumie S, Kuromatsu R, Okuda K, Ando E, Takata A, Fukushima N, Watanabe Y, Kojiro M, Sata M. Microvascular invasion in patients with hepatocellular carcinoma and its predictable clinicopathological factors. Ann Surg Oncol. 2008;15:1375–1382. doi: 10.1245/s10434-008-9846-9. [DOI] [PubMed] [Google Scholar]
- 15.Iguchi T, Shirabe K, Aishima S, Wang H, Fujita N, Ninomiya M, Yamashita Y, Ikegami T, Uchiyama H, Yoshizumi T, et al. New Pathologic Stratification of Microvascular Invasion in Hepatocellular Carcinoma: Predicting Prognosis After Living-donor Liver Transplantation. Transplantation. 2015;99:1236–1242. doi: 10.1097/TP.0000000000000489. [DOI] [PubMed] [Google Scholar]
- 16.Sun JJ, Wang K, Zhang CZ, Guo WX, Shi J, Cong WM, Wu MC, Lau WY, Cheng SQ. Postoperative Adjuvant Transcatheter Arterial Chemoembolization After R0 Hepatectomy Improves Outcomes of Patients Who have Hepatocellular Carcinoma with Microvascular Invasion. Ann Surg Oncol. 2016;23:1344–1351. doi: 10.1245/s10434-015-5008-z. [DOI] [PubMed] [Google Scholar]
- 17.Lam VW, Ng KK, Chok KS, Cheung TT, Yuen J, Tung H, Tso WK, Fan ST, Poon RT. Incomplete ablation after radiofrequency ablation of hepatocellular carcinoma: analysis of risk factors and prognostic factors. Ann Surg Oncol. 2008;15:782–790. doi: 10.1245/s10434-007-9733-9. [DOI] [PubMed] [Google Scholar]
- 18.Thanos L, Mylona S, Galani P, Pomoni M, Pomoni A, Koskinas I. Overcoming the heat-sink phenomenon: successful radiofrequency thermal ablation of liver tumors in contact with blood vessels. Diagn Interv Radiol. 2008;14:51–56. [PubMed] [Google Scholar]
- 19.Nault JC, Sutter O, Nahon P, Ganne-Carrié N, Séror O. Percutaneous treatment of hepatocellular carcinoma: State of the art and innovations. J Hepatol. 2017 doi: 10.1016/j.jhep.2017.10.004. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 20.McNaughton DA, Abu-Yousef MM. Doppler US of the liver made simple. Radiographics. 2011;31:161–188. doi: 10.1148/rg.311105093. [DOI] [PubMed] [Google Scholar]
- 21.Nicoli N, Casaril A, Abu Hilal M, Mangiante G, Marchiori L, Ciola M, Invernizzi L, Campagnaro T, Mansueto G. A case of rapid intrahepatic dissemination of hepatocellular carcinoma after radiofrequency thermal ablation. Am J Surg. 2004;188:165–167. doi: 10.1016/j.amjsurg.2003.12.061. [DOI] [PubMed] [Google Scholar]
- 22.Mori Y, Tamai H, Shingaki N, Moribata K, Shiraki T, Deguchi H, Ueda K, Enomoto S, Magari H, Inoue I, et al. Diffuse intrahepatic recurrence after percutaneous radiofrequency ablation for solitary and small hepatocellular carcinoma. Hepatol Int. 2009;3:509–515. doi: 10.1007/s12072-009-9131-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kotoh K, Nakamuta M, Morizono S, Kohjima M, Arimura E, Fukushima M, Enjoji M, Sakai H, Nawata H. A multi-step, incremental expansion method for radio frequency ablation: optimization of the procedure to prevent increases in intra-tumor pressure and to reduce the ablation time. Liver Int. 2005;25:542–547. doi: 10.1111/j.1478-3231.2005.01051.x. [DOI] [PubMed] [Google Scholar]
- 24.Seror O, N’Kontchou G, Nault JC, Rabahi Y, Nahon P, Ganne-Carrié N, Grando V, Zentar N, Beaugrand M, Trinchet JC, et al. Hepatocellular Carcinoma within Milan Criteria: No-Touch Multibipolar Radiofrequency Ablation for Treatment-Long-term Results. Radiology. 2016;280:611–621. doi: 10.1148/radiol.2016150743. [DOI] [PubMed] [Google Scholar]
- 25.Hocquelet A, Aubé C, Rode A, Cartier V, Sutter O, Manichon AF, Boursier J, N’kontchou G, Merle P, Blanc JF, et al. Comparison of no-touch multi-bipolar vs. monopolar radiofrequency ablation for small HCC. J Hepatol. 2017;66:67–74. doi: 10.1016/j.jhep.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 26.Song KD, Lee MW, Rhim H, Kim YS, Kang TW, Shin SW, Cho SK. Aggressive Intrasegmental Recurrence of Hepatocellular Carcinoma After Combined Transarterial Chemoembolization and Radiofrequency Ablation. AJR Am J Roentgenol. 2016;207:1122–1127. doi: 10.2214/AJR.16.16080. [DOI] [PubMed] [Google Scholar]
- 27.Hu KQ. Advances in clinical application of cryoablation therapy for hepatocellular carcinoma and metastatic liver tumor. J Clin Gastroenterol. 2014;48:830–836. doi: 10.1097/MCG.0000000000000201. [DOI] [PubMed] [Google Scholar]
- 28.Seifert JK, Stewart GJ, Hewitt PM, Bolton EJ, Junginger T, Morris DL. Interleukin-6 and tumor necrosis factor-alpha levels following hepatic cryotherapy: association with volume and duration of freezing. World J Surg. 1999;23:1019–1026. doi: 10.1007/s002689900617. [DOI] [PubMed] [Google Scholar]
- 29.Lee SM, Won JY, Lee DY, Lee KH, Lee KS, Paik YH, Kim JK. Percutaneous cryoablation of small hepatocellular carcinomas using a 17-gauge ultrathin probe. Clin Radiol. 2011;66:752–759. doi: 10.1016/j.crad.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 30.Wang C, Wang H, Yang W, Hu K, Xie H, Hu KQ, Bai W, Dong Z, Lu Y, Zeng Z, et al. Multicenter randomized controlled trial of percutaneous cryoablation versus radiofrequency ablation in hepatocellular carcinoma. Hepatology. 2015;61:1579–1590. doi: 10.1002/hep.27548. [DOI] [PubMed] [Google Scholar]
- 31.Rui J, Tatsutani KN, Dahiya R, Rubinsky B. Effect of thermal variables on human breast cancer in cryosurgery. Breast Cancer Res Treat. 1999;53:185–192. doi: 10.1023/a:1006182618414. [DOI] [PubMed] [Google Scholar]
- 32.Song KD, Lim HK, Rhim H, Lee MW, Kim YS, Lee WJ, Paik YH, Gwak GY, Kim JM, Kwon CH, et al. Repeated Hepatic Resection versus Radiofrequency Ablation for Recurrent Hepatocellular Carcinoma after Hepatic Resection: A Propensity Score Matching Study. Radiology. 2015;275:599–608. doi: 10.1148/radiol.14141568. [DOI] [PubMed] [Google Scholar]
- 33.Lubner MG, Brace CL, Hinshaw JL, Lee FT Jr. Microwave tumor ablation: mechanism of action, clinical results, and devices. J Vasc Interv Radiol. 2010;21:S192–S203. doi: 10.1016/j.jvir.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wright AS, Sampson LA, Warner TF, Mahvi DM, Lee FT Jr. Radiofrequency versus microwave ablation in a hepatic porcine model. Radiology. 2005;236:132–139. doi: 10.1148/radiol.2361031249. [DOI] [PubMed] [Google Scholar]
- 35.Smolock AR, Cristescu MM, Hinshaw A, Woo KM, Wells SA, Ziemlewicz TJ, Lubner MG, Dalvie PS, Louis Hinshaw J, Brace CL, et al. Combination transarterial chemoembolization and microwave ablation improves local tumor control for 3- to 5-cm hepatocellular carcinoma when compared with transarterial chemoembolization alone. Abdom Radiol (NY) 2018;43:2497–2504. doi: 10.1007/s00261-018-1464-9. [DOI] [PubMed] [Google Scholar]
- 36.Chinn SB, Lee FT Jr, Kennedy GD, Chinn C, Johnson CD, Winter TC 3rd, Warner TF, Mahvi DM. Effect of vascular occlusion on radiofrequency ablation of the liver: results in a porcine model. AJR Am J Roentgenol. 2001;176:789–795. doi: 10.2214/ajr.176.3.1760789. [DOI] [PubMed] [Google Scholar]
- 37.Charpentier KP, Wolf F, Noble L, Winn B, Resnick M, Dupuy DE. Irreversible electroporation of the liver and liver hilum in swine. HPB (Oxford) 2011;13:168–173. doi: 10.1111/j.1477-2574.2010.00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Niessen C, Igl J, Pregler B, Beyer L, Noeva E, Dollinger M, Schreyer AG, Jung EM, Stroszczynski C, Wiggermann P. Factors associated with short-term local recurrence of liver cancer after percutaneous ablation using irreversible electroporation: a prospective single-center study. J Vasc Interv Radiol. 2015;26:694–702. doi: 10.1016/j.jvir.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 39.Dollinger M, Müller-Wille R, Zeman F, Haimerl M, Niessen C, Beyer LP, Lang SA, Teufel A, Stroszczynski C, Wiggermann P. Irreversible Electroporation of Malignant Hepatic Tumors--Alterations in Venous Structures at Subacute Follow-Up and Evolution at Mid-Term Follow-Up. PLoS One. 2015;10:e0135773. doi: 10.1371/journal.pone.0135773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dollinger M, Beyer LP, Haimerl M, Niessen C, Jung EM, Zeman F, Stroszczynski C, Wiggermann P. Adverse effects of irreversible electroporation of malignant liver tumors under CT fluoroscopic guidance: a single-center experience. Diagn Interv Radiol. 2015;21:471–475. doi: 10.5152/dir.2015.14442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zimmerman A, Grand D, Charpentier KP. Irreversible electroporation of hepatocellular carcinoma: patient selection and perspectives. J Hepatocell Carcinoma. 2017;4:49–58. doi: 10.2147/JHC.S129063. [DOI] [PMC free article] [PubMed] [Google Scholar]


