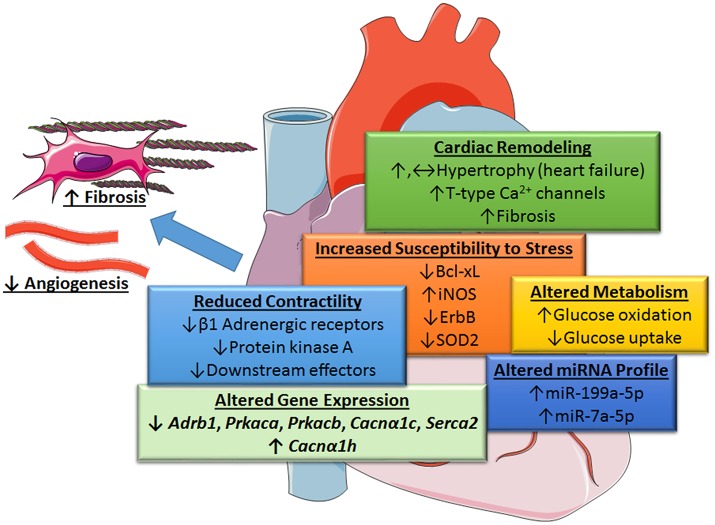
Figure 2.
Role of STAT3 in protecting the heart from increased blood pressure revealed by cardiac myocyte-targeted STAT3 deletion. Loss of cardiac STAT3 was found to reduce contractility from β1-adrenergic stimulation and increase susceptibility of the heart to stress by altering gene expression. Under basal conditions, the following genes are downregulated in cardiomyocyte-targeted hearts (↓): β1 adrenergic receptor (Adrb1), protein kinase A (PKA) catalytic subunit α and β (Prkaca and Prkacb), L-type calcium channel subunit (Cacnα1c), and Serca2; whereas, α-subunit of voltage-gated T-type Ca2+ channel (Cacnα1h) is upregulated (↑), Enhanced levels of certain microRNAs (miRNA-199a-5p and miRNA-7a-5p) induced oxidative stress due to altered glucose metabolism under stress conditions and diminished protective neuregulin-ErbB signaling. Overall, loss of cardiac myocyte STAT3 has a neutral or positive effect (possibly through an increase in T-type Ca2+ channels and NFAT activation) on cardiac hypertrophy and associated fibrosis. Evidence suggests, however, that STAT3-deficient cardiac myocytes produce unidentified paracrine factors that attenuate capillary formation and promote fibrosis with aging. Images adapted from Servier Medical Art (https://smart.servier.com/).