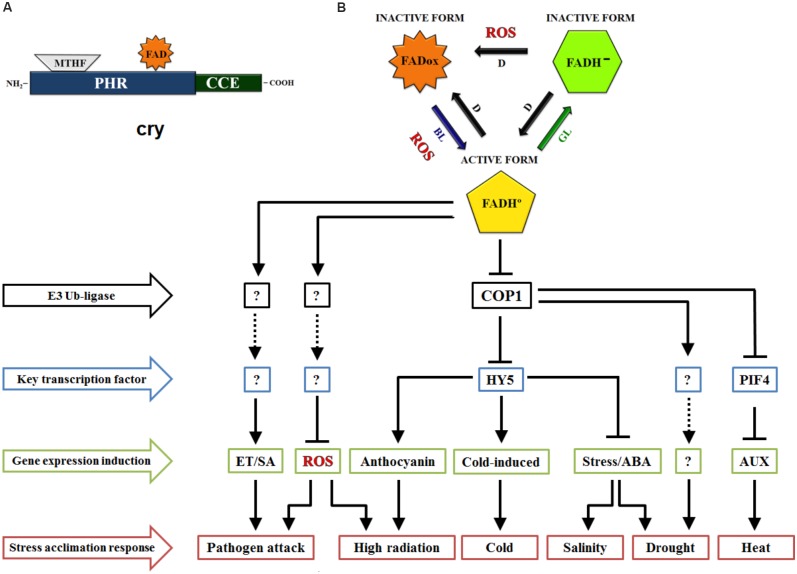
FIGURE 1.
Schematic view of cryptochromes (cry) role in stress acclimation responses. (A) Domain structure of cry consists of Photolyase-Homologous Region (PHR) domain and CRY C-terminal extension (CCE) domain. The cry chromophores methenyltetrahydrofolate (MTHF) and FAD are binding to PHR, a light perception domain. (B) FAD chromophore redox states of cry. FAD is oxidized (FADox) in the darkness (D), with the absorption peak in blue light (BL). On light exposure, FADox changes to the neutral semireduced state (FADH°), which allows the biological activity of cry in the plant cell. With the absorption peak in green light (GL), FADH° is induced to change to the totally reduced form (FAD-). An unclear subject around the reoxidation of FAD is the production of reactive oxygen species (ROS) during the process. Stress signaling pathway regulated by cryptochromes. From the blue light signaling, cry coordinate the negative regulation of COP1-dependent degradation of HY5 and PIF4 transcription factors, triggering changes in stress response target genes expression. Note that these are key pathways in the induction of genes related to signaling and/or biosynthesis of hormones, ROS and stress components. Although the complex signaling of cry in stress acclimation still involves some unknown components, certainly this photoreceptor play a crucial role in BL-dependent stress responses. Arrows means downregulation, T-bars indicate upregulation and dotted lines indicate unknown (?) signaling routes. E3 ubiquitin-ligase; COP1, constitutive photomorphogenic 1; HY5, long hypocotyl 5; PIF4, phytochrome interacting factor 4; ET, ethylene; SA, salicylic acid; ABA, abscisic acid; AUX, auxin.