Abstract
The antral follicle count (AFC) is used as an indicator of cow fertility. We herein investigated the relationship between AFC and the steroidogenesis of granulosa cells and confirmed the developmental competence of oocytes derived from early antral follicles (0.5–1.0 mm) using in vitro growth culture. Slaughterhouse-derived ovaries were divided into high (≥ 25) and low (< 25) AFC groups based on AFC (≥ 2.0 mm). Oocyte-cumulus-granulosa complexes (OCGCs) collected from early antral follicles were cultured for 12 days. The total number, viability, and diameter of granulosa cells and estradiol-17β and progesterone production during the culture were evaluated. Surviving oocytes on day 12 were subjected to in vitro maturation, and their volume and nuclear status were evaluated. Some oocytes were subjected to the evaluation of developmental competence to blastocysts. Although the total number and viability of granulosa cells did not differ between the groups, granulosa cell diameters were smaller in the high AFC group than in the low AFC group. The estradiol-17β and progesterone ratio on day 8 was higher in the high AFC group than in the low AFC group. Oocyte volumes and nuclear maturation rates were greater in the high AFC group than in the low AFC group. The development rate to blastocysts was 9.1% in the high AFC group, while no oocytes developed to blastocysts in the low AFC group. Therefore, estradiol-17β production by granulosa cells appears to be greater in high AFC cattle than in low AFC cattle, thereby promoting the acquisition of oocyte competence.
Keywords: Antral follicle count, Bovine oocyte, In vitro growth, Steroidogenesis
The primary roles of the ovaries are to support the growth and maturation of oocytes for the acquisition of fertilizability and developmental competence, as well as the production of sex steroid hormones for inducing the estrous cycle and sustaining pregnancy. The ovarian reserve, the number of primordial follicles in a pair of ovaries in individuals, is defined as the potential ability of these functions [1] and is known to be an indicator of female fertility in humans [2] and cattle [3]. The number of antral follicles in a pair of ovaries counted by ultrasonography (the antral follicle count; AFC) positively correlates with the number of primordial follicles [4] and may be used to estimate the ovarian reserve [5]. Although AFC fluctuates during the estrus cycle and markedly varies between individuals, peak AFC during the estrous cycle shows high repeatability in individual cattle [5]. Between 15 and 20% of individual cattle in a herd were generally classified into the low AFC group (15 or fewer follicles), while 15–20% were in the high AFC group (25 or greater follicles), and the remainder were in the intermediate group (16–24 follicles) [3, 5]. High AFC cattle with 25 or more follicles in a pair of ovaries showed higher reproductive performance, such as greater fertility [6], a shorter open period [6], and greater responsiveness to superovulation [7], than low AFC cattle having 15 or fewer follicles, even though they were in the same age class.
We previously reported that the fertilizability of oocytes collected by ultrasound-guided ovum-pick up (OPU) was greater in high AFC cattle with 30 or more follicles in a pair of ovaries than low AFC cattle with less than 30 follicles at a 3- or 4-day interval of OPU [8]. In contrast, when we extended the interval of OPU to 7 days, the fertilizability of oocytes in high AFC cattle was impaired and became less than that in low AFC cattle [8]. These findings indicate that the growth dynamics of antral follicles differ between high and low AFC cattle, and the degeneration of antral follicles at the selection phase in the follicular wave may occur earlier in high AFC cattle than in low AFC cattle. To investigate the differences underlying follicular growth dynamics and the acquisition of oocyte competence between high and low AFC cattle, we conducted a study using an in vitro growth (IVG) culture of bovine oocytes [9], a culture system that enables bovine oocytes without maturational competence from early antral follicles to grow to the stage acquiring competence for maturation and development to the blastocyst stage [10,11,12]. Consequently, oocyte-cumulus-granulosa complexes (OCGCs) derived from early antral follicles (0.5–1.0 mm in diameter) in the high AFC group with more than 25 follicles (≥ 2.0 mm in diameter) in an ovary collected at a slaughterhouse showed greater oocyte maturational competence and fertilizability as well as the greater proliferation of granulosa cells than those in the low AFC group (less than 25 follicles) [9]. However, in the previous study [9], we cultured OCGCs in medium containing a high concentration of estradiol-17β (E2, 1 µg/ml) to increase the E2/progesterone (P4) ratio, similar to a dominant follicle [13]; therefore, the relationships between AFC, the steroidogenesis of granulosa cells, and the developmental competence of oocytes have not yet been examined. In addition, we reported that granulosa cells surrounding in vitro-grown oocytes having higher maturational competence secreted slightly more E2 and less P4 than the granulosa cells surrounding less competent in vitro-grown oocytes using medium containing androstenedione (A4) instead of E2 [14].
In the present study, we investigated the relationship between AFC and the steroidogenesis of granulosa cells using a bovine IVG culture without the exogenous application of E2. In addition, we confirmed the oocyte competence of growth, maturation, and subsequent development to blastocysts.
Materials and Methods
Chemicals
All the chemicals used in the present study were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise stated.
Collection of OCGCs and the IVG culture
Ovaries of Holstein cows obtained from a local abattoir were stored in plastic bags at 20°C and transported to the laboratory within 6–10 h of their collection. After the ovaries were washed three times with physiological saline, slices of ovarian cortex tissues (thickness < 1 mm) were prepared using a surgical blade (no. 11) and stored in tissue culture medium 199 (TCM-199; Thermo Fisher Scientific, Roskilde, Denmark) supplemented with 0.1% polyvinyl alcohol, 25 mM 2-[4-(2-Hydroxyethyl)-1-piperazinyl] ethanesulfonic acid (HEPES), 10 mM sodium bicarbonate, and 50 μg/ml gentamicin sulfate (isolation medium, pH 7.4) at 37°C, as described elsewhere [15]. Under a stereomicroscope, early antral follicles (0.5–1.0 mm in diameter) were dissected from sliced ovarian tissues using a surgical blade (no. 20) and fine forceps in a 90-mm petri dish that had a 1-mm scale on its bottom (FLAT, Chiba, Japan). OCGCs were isolated from early antral follicles using a pair of fine forceps and subjected to IVG as previously described [14]. Growth medium was HEPES-buffered TCM-199 supplemented with 0.91 mM sodium pyruvate, 5% (v/v) fetal calf serum (FCS; Invitrogen), 4 mM hypoxanthine, 4% (w/v) polyvinylpyrrolidone (MW 360,000), 50 μg/ml ascorbic acid 2-glucoside (Wako Pure Chemical Industries, Osaka, Japan), 55 μg/ml cysteine, 50 μg/ml gentamicin sulfate, and 10 ng/ml A4 as a precursor for E2. OCGCs with oocytes surrounded by a cumulus investment and attached mural granulosa-cell layer (Fig. 1A) were cultured individually in a 96-well culture plate (Primaria 353872, Corning Life Sciences, Tewksbury, MA, USA) with 200 μl of growth medium at 39°C for 12 days in humidified air with 5% CO2. At the onset of the IVG culture, OCGCs were photographed under an inverted microscope (CK 40, Olympus, Tokyo, Japan) with an attached CCD camera (Moticam 2000, Shimadzu Rika, Tokyo, Japan). The diameters of the oocytes without a zona pellucida were assessed using software (Motic Images Plus 2.2s, Shimadzu). Every 4 days of the IVG culture, half (100 μl) of the growth medium was replaced with the same amount of fresh medium. Spent media collected on days 4, 8, and 12 of the culture were stored at –30°C until steroid hormone assays were conducted.
Fig. 1.
Oocyte-cumulus-granulosa complexes (OCGCs) before and after day 12 of the growth culture. A: An OCGC before the growth culture. The OCGC has an oocyte surrounded by a cumulus investment and attached mural granulosa-cell layer. The white arrowhead indicates the cumulus investment. The black arrowhead indicates the mural granulosa-cell layer. B: A surviving OCGC without antrum formation in the granulosa cell layer after a 12-day growth culture. OCGCs having oocytes with an evenly granulated ooplasm and enclosed by several layers of healthy granulosa cells were defined as surviving. C: A dead OCGC having a degenerated oocyte. D: A surviving OCGC with the formation of antra (white arrowheads) in the granulosa cell layer. The white arrow indicates an oocyte. Scale bars indicate 100 µm.
Evaluation of OCGC morphologies
Every 4 days of the IVG culture, the viability of OCGCs was assessed by their morphological appearance [14]; i.e., OCGCs having an evenly granulated ooplasm that was completely enclosed by several layers of a healthy cumulus and granulosa cells were defined as surviving (Fig. 1B). OCGCs having oocytes with an abnormal appearance and/or denuded by a scattering cumulus and granulosa cells were defined as dead (Fig. 1C). Simultaneous antrum formation in granulosa cell layers (Fig. 1D), which was related to a high ability for E2 production [14, 16], was also recorded.
E2 and P4 assays
Spent media (100 μl) out of 200 μl of growth media was assayed to assess E2 and P4 concentrations using a competitive double antibody enzyme immunoassay, as previously described [17]. Samples were subjected to 2- to 2000-fold serial dilutions with assay buffer (145 mM NaCl, 40 mM Na2HPO4, and 0.1% bovine serum albumin (BSA, w/v, pH 7.2)). Diluted samples (20 μl) were incubated with the primary antisera and horseradish peroxidase-labeled hormone (100 μl each) in the wells of a 96-well microplate (Costar 3590, Corning, NY, USA) coated with the secondary antiserum at 4°C for 16–18 h. The primary antisera used for the E2 and P4 assays were anti-estradiol-17β-6-carboxymethyloxime (CMO)-BSA (FKA204, Cosmo bio, Tokyo, Japan) and anti-progesterone-3-CMO-BSA (KZ-HS-P13, Cosmo bio), respectively. Goat anti-rabbit serum (111-005-003, Jackson Immuno Research, West Grave, PA, USA) was used as the secondary antiserum. After the washing of all wells four times with 300 μl of washing buffer (0.05% Tween 80), 150 μl of 3,3',5,5'-tetramethylbenzidine (TMB) solution (5 mM citric acid, 50 mM Na2HPO4, 500 mM urea hydrogen peroxide, 1 mM TMB, and 2% dimethyl sulfoxide) was added to each well and incubated at 37°C for 40 min. The absorbance of the solution in the wells was measured at 450 nm using a microplate reader (Model 550, Bio-Rad Laboratories, Tokyo, Japan) after stopping the chromogenic reaction with 50 μl of 4 N H2SO4. All samples were assayed in triplicate. Assay sensitivities were 7.1 pg/well for E2 and 11.2 pg/well for P4. The inter- and intra-assay coefficients of variations were 16.9 and 4.0% for E2 and 7.0 and 3.9% for P4, respectively.
Evaluation of granulosa cell characteristics
The total number, viability, and diameter of granulosa cells after the growth culture from morphologically normal OCGCs on days 8 and 12 were assessed using an acridine orange/propidium iodide cell viability kit together with a cell counter (F23001 and L2000, respectively; Logos Biosystems, Gyunggi, Republic of Korea) as previously described [18]. The culture medium in the well of each viable OCGC was removed and replaced with 80 μl of Dulbecco’s phosphate-buffered saline without calcium and magnesium (DPBS) supplemented with 0.125% (w/v) trypsin and 0.05% (w/v) EDTA to prepare the granulosa cells for counting. After 10 min of trypsinization and pipetting several times, 20 μl of FCS was added to stop digestion. The cell counter calculated the viability and gave a mean diameter of granulosa cells used 21 to 1123 cells/sample in the present study. The denuded oocyte was removed from the well and discarded.
Evaluation of the growth, nuclear maturation, and developmental competence of oocytes
After 12 days of the IVG culture, in vitro maturation (IVM) was performed as previously described [19]. Briefly, oocytes surrounded by several layers of cumulus cells (oocyte-cumulus complexes: COCs) from surviving OCGCs were washed with IVM medium, which consisted of HEPES-buffered TCM-199 supplemented with 0.2 mM sodium pyruvate, 20 μg/ml follicle-stimulating hormone (FSH), 1 μg/ml E2, 10% FCS, and 50 μg/ml gentamicin sulfate. COCs were cultured in each well of micro-well plates filled with 6 ml of IVM medium at 39°C under 5% CO2 in air for 22 h. After IVM, oocytes were denuded from cumulus cells by individual pipetting, photographed, and their diameters were measured. Oocyte volumes were calculated by their diameters. Oocytes were mounted individually on a slide glass and fixed with a mixture of acetic acid and ethanol (1:3) overnight. After fixation, oocytes were stained with 1% (w/v) aceto-orcein and the statuses of their nuclei were examined under a phase contrast microscope, as described elsewhere [20]. Oocytes at metaphase II and having a polar body were defined as mature; oocytes at other nuclear statuses were defined as immature. After IVM, some COCs were subjected to in vitro fertilization (IVF) as previously described [21]. Briefly, frozen semen collected from a Holstein bull was used for IVF. After thawing the semen in a 37°C water bath for 40 sec, motile sperm (5 × 106 sperm/ml) separated by a Percoll gradient (45% and 90%) were co-incubated with COCs in a 100-μl droplet (8 to 12 COCs per droplet) of modified Brackett and Oliphant isotonic medium [22] containing 3 mg/ml fatty acid–free BSA and 2.5 mM theophylline [23] for 18 h at 39°C in a humidified atmosphere of 5% CO2, 5% O2, and 90% N2. An in vitro culture (IVC) of inseminated oocytes (presumptive zygotes) was performed as previously described [21, 24]. Briefly, after a co-incubation with sperm, presumptive zygotes were freed from cumulus cells by vortexing for 5 min and washing three times in culture medium. Cumulus-free zygotes were cultured for 6 days in 30-μl droplets (8 to 12 zygotes per droplet) of culture medium at 39°C under 5% CO2, 5% O2, and 90% N2. The culture medium consisted of modified synthetic oviduct fluid containing 1 mM glutamine, 12 essential amino acids for basal medium Eagle, seven non-essential amino acids for minimum essential medium, 10 μg/ml insulin, 5 mM glycine, 5 mM taurine, 1 mM glucose, and 3 mg/ml fatty acid–free BSA. During IVF and IVC, oocytes derived from different ovaries were pooled and cultured in a same droplet. Cleavage and blastocyst production rates were measured after 2 days (approximately 30 h) and 6 days (150 h) of IVC, respectively. The total live cell numbers of blastocysts obtained after 6 days of IVC were counted using an air-drying method [23].
Statistical analysis
All statistical analyses were performed using software (StatView 4.51, Abacus Concepts, Calabasas, CA, USA). Data on the viability of and antrum formation by OCGCs and the nuclear maturation rate were analyzed by the chi-squared test. Other data were analyzed using a two-way ANOVA followed by the Student’s t-test or Tukey-Kramer’s HSD test.
Experimental design
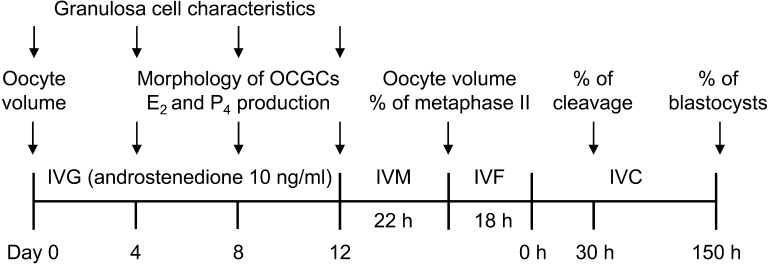
A schematic of the experimental design was shown in Fig. 2. AFC in ovaries from the local abattoir was assessed by the number of antral follicles (≥ 2 mm in diameter), and we allocated ovaries having 25 or more follicles to the high AFC group and others to the low AFC group as described elsewhere [9]. As shown in Fig. 3, we collected more OCGCs from a higher number of antral follicles (≥ 2 mm in diameter) in ovaries (P < 0.05, Tukey-Kramer’s HSD test). We used 186 ovaries as the low AFC group and 70 ovaries as the high AFC group. We collected 388 OCGCs in the low AFC group and 410 OCGCs in the high AFC group and subjected them to each experiment. However, as shown in Table 1, 4 oocytes in the low AFC group and 4 in the high AFC group were accidentally lost after IVM during the pipetting of oocytes for the denudation of cumulus cells. Three oocytes in the low AFC group and 2 oocytes in the high AFC group were accidentally lost after IVF during the vortexing procedure (Table 1). As shown in Table 1, 193 OCGCs in the low AFC group and 225 in the high AFC group were subjected to IVG followed by IVM for the evaluation of oocyte volumes on day 0 of IVG and after IVM and the percentage of metaphase II oocytes after IVM. E2 and P4 concentrations were measured in spent media used for the IVG culture of 95 OCGCs in the low AFC group and 110 OCGCs in the high AFC group, which were subjected to IVM. Steroid hormone production during each period (days 0 to 4, 4 to 8, and 8 to 12) was calculated based on concentrations at the beginning and end of the period as previously described [14]. After IVM, 44 OCGCs in low AFC group and 44 OCGCs in high AFC group were subjected to IVF and IVC for the evaluation of developmental competence. As shown in Table 2, the remaining 151 OCGCs in the low AFC group and 141 OCGCs in the high AFC group were subjected to an evaluation of the characteristics of granulosa cells on days 0, 4, 8, and 12. The viability of OCGCs was calculated based on all cultured OCGCs. The percentage of antrum formation in the granulosa cell layer was calculated based on OCGCs judged as surviving on day 12. We compared differences in the viability of OCGCs, antrum formation in the granulosa cell layer, the total number, viability, and diameter of granulosa cells, production of E2 and P4, the E2/P4 ratio, and volume, nuclear status, and developmental competence of oocytes between the AFC groups.
Fig. 2.
Schematic of the experimental design. AFC in an ovary from the local abattoir was assessed by the number of antral follicles (≥ 2 mm in diameter), and we allocated ovaries having 25 or more follicles to the high AFC group and others to the low AFC group. OCGCs from each ovary were cultured for 0, 4, 8, or 12 days in an in vitro growth (IVG) culture. Oocyte volume was evaluated on day 0 of IVG. Granulosa cell characteristics were evaluated on every 4 days of IVG (days 0, 4, 8, and 12). On every 4 days of IVG, the morphology of OCGCs (viability of OCGCs and antrum formation in granulosa cell layers) was evaluated. After 12 days of IVG, oocyte-cumulus complexes (COCs) derived from surviving OCGCs were subjected to in vitro maturation (IVM). The concentrations of estradiol-17β (E2) and progesterone (P4) in the IVG media of some of these OCGCs at each period of IVG every 4 days (days 0, 4, 8, and 12) were evaluated. After IVM, the volume and nuclear status of some oocytes were evaluated. The remaining oocytes were subjected to in vitro fertilization (IVF) and an in vitro culture (IVC) for the evaluation of developmental competence to blastocysts.
Fig. 3.
Relationship between AFC and number of collected OCGCs in each ovary. A total of 186 ovaries as the low AFC group and 70 ovaries as the high AFC group were used, and 388 OCGCs in the low AFC group and 410 OCGCs in the high AFC group were collected. a–f Different letters indicate significant differences between different AFC (P < 0.05). Error bars indicate SEM.
Table 1. Number of oocyte-cumulus-granulosa complexes (OCGCs) cultured for the evaluation of nuclear maturation, production of estradiol-17β (E2) and progesterone (P4), and developmental competence.
| Groups | No. of OCGCs used for oocyte volume and nuclear maturation |
No. of OCGCs used for oocyte developmental competence |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Cultured | Survived | Evaluated | E2 and P4 measurement | Cultured | Survived | Evaluated | Cleaved | Blastocysts | |
| (%) | (%) | ||||||||
| Low | 193 | 111 | 107 a | 95 | 44 | 22 | 19 b | 12 (63.2) | 0 (0.0) |
| High | 225 | 137 | 133 a | 110 | 44 | 24 | 22 c | 15 (68.2) | 2 (9.1) |
a Four oocytes were lost during pipetting after in vitro maturation. b Three oocytes were lost during vortexing after in vitro fertilization. c Two oocytes were lost during vortexing after in vitro fertilization. The numbers in parentheses indicate rates of cleaved oocytes or oocytes developed to blastocysts after in vitro fertilization and in vitro culture. The cell numbers in blastocysts were 65 and 88 in the high AFC group.
Table 2. Number of oocyte-cumulus-granulosa complexes (OCGCs) cultured for the evaluation of granulosa cell characteristics.
| Groups | Total | No. of OCGCs used for granulosa characteristics at each duration |
||||||
|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 4 |
Day 8 |
Day 12 |
|||||
| Cultured | Survived | Cultured | Survived | Cultured | Survived | |||
| Low | 151 | 26 | 34 | 32 | 38 | 27 | 53 | 25 |
| High | 141 | 27 | 27 | 23 | 37 | 24 | 50 | 28 |
Results
Relationships between AFC, viability, and antrum formation of OCGCs
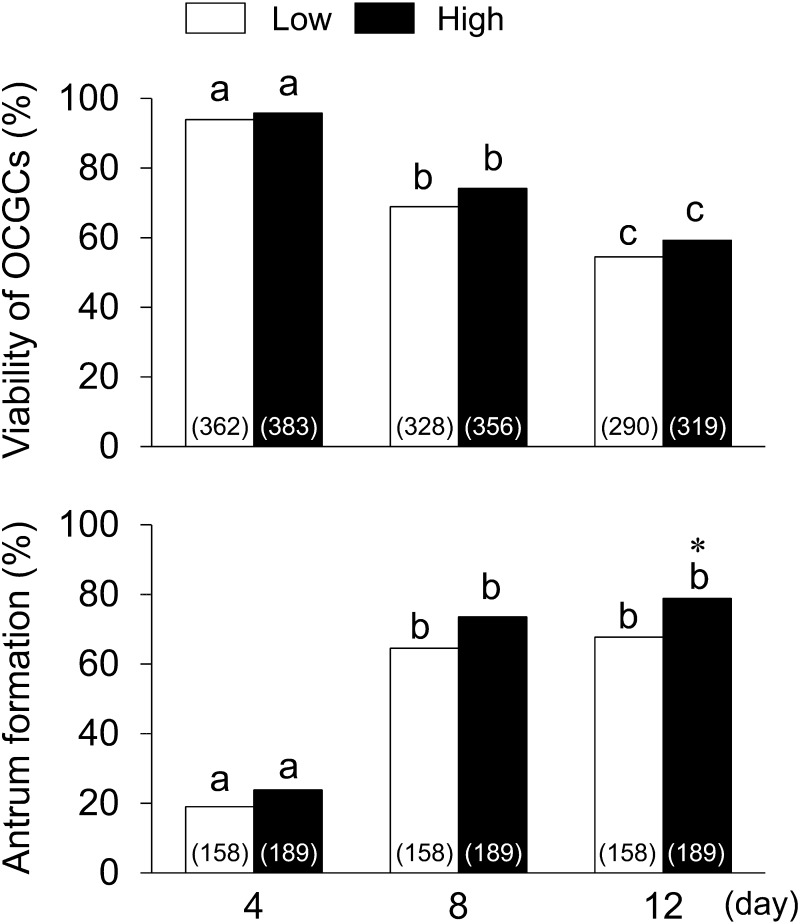
As shown in Fig. 4, the viabilities of OCGCs in the high and low AFC groups decreased throughout the culture period (P < 0.05) and did not significantly differ between the two groups. The percentages of OCGCs having antra increased throughout the culture period, and became higher in the high AFC (78.8%) group than in the low AFC group (67.7%, P < 0.05) on day 12.
Fig. 4.
Relationships between AFC, viability, and antrum formation in granulosa cell layers of OCGCs. Numbers in parentheses indicate the number of OCGCs; namely, the number of OCGCs in which viability was evaluated on day 12 is a summary of OCGCs cultured for the evaluation of nuclear maturation (low AFC: 193, high AFC: 225) and developmental competence (low AFC: 44, high AFC: 44) in Table 1, and granulosa cell characteristics on day 12 (low AFC: 53, high AFC: 50) in Table 2. The number of OCGCs in which viability was evaluated on day 8 was a summary of OCGCs evaluated as surviving on day 12 and OCGCs evaluated granulosa cell characteristics on day 8 (low AFC: 38, high AFC: 37) in Table 2. The number of OCGCs in which viability was evaluated on day 4 (low AFC: 362, high AFC: 383) was the summary of OCGCs evaluated as surviving at day 8 and OCGCs evaluated granulosa cell characteristics on day 4 (low AFC: 34, high AFC: 27) in Table 2. As shown in Tables 1 and 2, the number of OCGCs in which antrum formation was evaluated (low AFC: 158, high AFC: 189) was the sum of those surviving on day 12 that were used for the evaluation of nuclear maturation (low AFC: 111, high AFC: 137), developmental competence (low AFC: 22, high AFC: 24), and granulosa cell characteristics on day 12 (low AFC: 25, high AFC: 28). a–c Different letters indicate significant differences between different culture periods in the same group (P < 0.05). * An asterisk indicates a significant difference between the low and high AFC groups on the same day (P < 0.05).
Relationship between AFC and the steroidogenesis of granulosa cells
As shown in Fig. 5, the two-way ANOVA showed that the interactions between AFC and the culture duration on E2 production and the E2/ P4 ratio (P < 0.01), and E2 production and the E2/P4 ratio were affected by AFC (P < 0.05) and the culture duration (P < 0.01). P4 production was affected by the culture duration, but not by AFC. E2 production from days 4 to 8 showed the highest values in all culture periods, and E2 production from days 4 to 8 was higher in the high AFC group than in the low AFC group (P < 0.05). P4 production increased with the extension of the culture period (P < 0.05), and did not significantly differ between the two groups. The E2/ P4 ratio in the high AFC group did not decrease until day 8, and was higher than that in the low AFC group (P < 0.05) on day 8; however, the E2/ P4 ratio decreased with the extension of the culture period (P < 0.05) in the low AFC group.
Fig. 5.
Relationships between antral follicle counts (AFC), the production of estradiol-17β (E2) and progesterone (P4) by oocyte-cumulus-granulosa complexes (OCGCs), and the E2/P4 ratio in culture media. a–c Different letters indicate significant differences between different culture periods in the same group (P < 0.05). * An asterisk indicates a significant difference between the low and high AFC groups (P < 0.05). Numbers in parentheses indicate the number of OCGCs on the same day. Error bars indicate SEM.
Relationship between AFC and granulosa cell characteristics
As shown in Fig. 6, the two-way ANOVA showed that the culture duration affected the total number, viability, and diameter of granulosa cells (P < 0.01) and AFC affected the diameter of granulosa cells (P < 0.05); however, there were no interactions between AFC and the culture duration on the total number, viability, and diameter of granulosa cells. The total number of granulosa cells increased throughout the culture period (P < 0.05) and did not significantly differ between the two groups. The viability of granulosa cells was higher than 97% throughout the culture and similar between the two groups. The mean diameters of granulosa cells were larger in the low AFC group than in the high AFC group during all culture periods (P < 0.05), and became larger on day 4 of the culture than that on day 0 (before IVG) (P < 0.05).
Fig. 6.
Relationships between antral follicle counts (AFC) and the total number, viability, and diameter of granulosa cells of oocyte-cumulus-granulosa complexes (OCGCs). a–c Different letters indicate significant differences between different culture periods in the same group (P < 0.05). * An asterisk indicates a significant difference between the low and high AFC groups on the same day (P < 0.05). Numbers in parentheses indicate the number of OCGCs. Error bars indicate SEM.
Relationships between AFC, growth, maturation, and developmental competence of oocytes
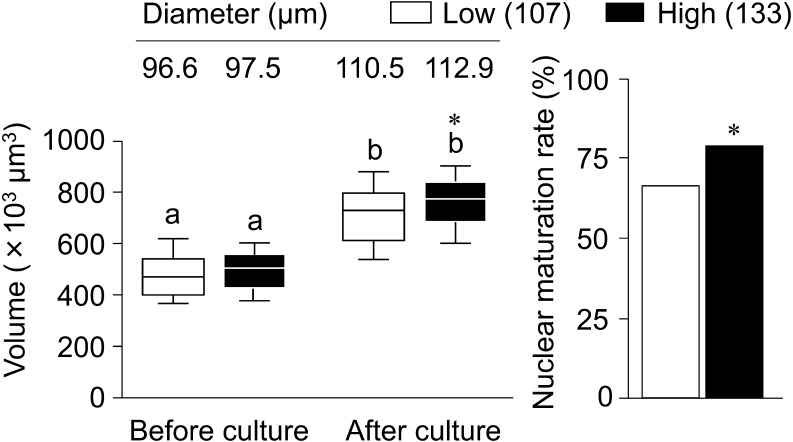
As shown in Fig. 7, the two-way ANOVA showed that IVG culture and AFC affected the volume of oocytes (P < 0.01); however, there was no interaction between AFC and IVG culture on the volume of oocytes. The volume of oocytes became larger after than before IVG in each group (P < 0.05), and the high AFC group showed a larger volume than the low AFC group after IVG (P < 0.05). The nuclear maturation rate was higher in the high AFC group (78.9%, 105/133) than in the low AFC group (66.4%, 71/107) (P < 0.05).
Fig. 7.
Relationships between antral follicle counts (AFC) and oocyte growth and maturation. Lines on the boxes in the box-and-whisker plot delineate the 25th, 50th, and 75th percentiles, while the whiskers depict the 10th and 90th percentiles. Values above boxes in the box-and-whisker plot indicate the mean diameters (μm) of oocytes. Numbers in parentheses indicate the number of oocytes submitted to IVM (Table 1) derived from 193 OCGCs in the low AFC group and 225 in the high AFC group. a, b Different letters indicate significant differences between before and after IVG (P < 0.05). * An asterisk indicates a significant difference between the low and high AFC groups on the same day (P < 0.05).
As shown in Table 1, cleavage rates were similar between the low AFC (63.2%, 12/19) and the high AFC group (68.2%, 15/22), but no oocytes (0/19) in the low AFC group developed to the blastocyst stage after IVF and IVC, while 9.1% (2/22) oocytes in the high AFC group developed to blastocysts.
Discussion
In the present study, E2 production was greater in the high AFC group than in the low AFC group. Furthermore, we confirmed a higher nuclear maturation rate and blastocyst development in the high AFC group. The present results on oocyte competence support our previous findings showing that oocytes derived from early antral follicles in ovaries with high AFC had a greater maturational ability, and were assumed to have greater fertilizability than ovaries with low AFC [9]. Moreover, the present study suggests that greater E2 production and a higher E2/P4 ratio contributed to superior oocyte competence in the high AFC group.
Granulosa cell numbers and viabilities were similar between the two groups in the present study; however, the proliferation of granulosa cells was greater in the high AFC group than in the low AFC group in our previous study [9]. We speculate that this difference in granulosa cell proliferation may be related to the addition of different steroid hormones to culture media in the two studies. We used a high concentration of E2 in the previous study [9], whereas we used A4 in the present study. Taketsuru et al. [25] reported that the viability of OCGCs was approximately 80% when OCGCs were cultured in E2-supplemented medium for 14 days, but approximately 65% when OCGCs were cultured in A4-supplemented medium. These findings suggest that the effects of A4 on the enhancement of granulosa cell proliferation were weaker than those of E2. The mean number of granulosa cells was 8.5 × 104 cells after 12 days of IVG in our previous study using E2-supplemented medium [18], but were 5.6 × 104 and 6.6 × 104 cells in the low and high AFC groups, respectively, in the present study. Although our previous study evaluating the effects of AFC on the granulosa cell number using E2-supplemented medium [9] showed lower values (approximately 4.0–5.0 × 104 cells) than the present results, we collected and counted granulosa cells after retrieving COCs [9], thereby reducing the number of remaining granulosa cells in a well.
The mean diameter of granulosa cells was larger in the low AFC group (11.3 ± 0.4 µm) than in the high AFC group (10.2 ± 0.3 µm) even before IVG. Previous studies reported that in vivo-grown large luteal cells (38.4 µm in diameter) originated from granulosa cells and in vitro-luteinized granulosa cells (38.4 µm) were larger than granulosa cells in preovulatory follicles (10.6 µm) [26, 27]. In addition, Scheetz et al. [28] suggested that P4 production and the expression level of the oxytocin-neurophysin I precursor, both of which are markers of granulosa cell luteinization, were higher in granulosa cells from low AFC ovaries having 15 or fewer follicles in a pair of ovaries than from high AFC ovaries having 25 or greater follicles; however, they did not measure the diameter of granulosa cells. The difference in the diameter of granulosa cells may support luteinization or luteinization-like changes in the low AFC group, although P4 production was not significantly different between both groups in the present study. Endo et al. [16] reported that E2 promoted the growth and maturational competence of oocytes derived from early antral follicles using bovine IVG. Moreover, our previous findings [14] suggested that E2 production by OCGCs producing matured oocytes after IVM was slightly higher than that of OCGCs producing immature oocytes after IVM. In the present study, the development rate to blastocyst was 9.1% in the high AFC group, and the developmental competence was similar to that of bovine oocytes with < 115 µm in diameter (11.9%) in our previous study [29]. On the other hand, we could not produce blastocysts from the oocytes in the low AFC group, although the cleavage rate after IVF was similar between both groups. The result may indicate the impaired developmental competence of oocytes in the low AFC group. In our previous study [9], some oocytes in the low AFC group developed to blastocysts when we used E2 (1 µg/ml) for the growth medium instead of A4 to increase the E2/P4 ratio like a dominant follicle [13]. E2 addition may enhance the granulosa cell proliferation because the numbers of granulosa cells were relatively greater in the previous study (8.5 × 104 cells/well at day 12) [18] compared to the present results, 5.6 and 6.6 × 104 cells/well in the low and high AFC groups, respectively. These results suggest that the impaired E2 production of granulosa cells in the low AFC group had a negative impact on oocyte growth, maturation, and developmental competence. In addition, we performed 10-h culture of in vivo-grown oocytes with low FSH containing medium before IVM culture (pre-IVM) in the previous study [9]. Also, we reported that pre-IVM improved the developmental competence of bovine oocytes derived from IVG [11] and oocytes with < 115 µm in diameter [29]. In further study, we should examine the effects of E2/P4 ratio during IVG culture and FSH treatment before IVM on the acquisition of developmental competence of in vitro-grown oocytes.
Scheetz et al. [28] also reported that FSH-mediated E2 production was lower in granulosa cells from low AFC ovaries than from high AFC ovaries by culturing granulosa cells under serum-free conditions. However, we previously demonstrated that the addition of FSH did not enhance E2 production and accelerated P4 production [14], which may have been due to the addition of serum to the medium for the IVG culture. Granulosa cells cultured in media containing serum were found to have luteinized, compromised E2 production, and begun to produce P4 [30, 31]. Previous studies attempted to culture OCGCs in serum-free media [32, 33]; however, the oocytes derived from serum-free cultures had low maturational competence and low fertilizability. In the future, we need to develop an IVG system that does not use serum, but enhances oocyte competence, or that inhibits the luteinization of granulosa cells even when growth medium contains serum.
In conclusion, E2 production by bovine granulosa cells cultured as OCGCs was greater in the high AFC group than in the low AFC group, whereas P4 production by granulosa cells was similar in each group. The diameter of granulosa cells was larger in the low AFC group than in the high AFC group. These results indicate that granulosa cells in the low AFC group are starting luteinization, and the reduced production of E2 by granulosa cells in the low AFC group may impair the growth and meiotic competence of oocytes. In future studies, we need to identify possible factors inducing luteinization-like changes in the low AFC group.
Acknowledgments
This work was supported by JSPS KAKENHI Grant Number JP16K08043 to MN. Sakaguchi K was supported by JSPS Research Fellowships for Young Scientists. We thank the Genetics Hokkaido Association for the donation of frozen bull sperm.
References
- 1.te Velde ER, Pearson PL. The variability of female reproductive ageing. Hum Reprod Update 2002; 8: 141–154. [DOI] [PubMed] [Google Scholar]
- 2.Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update 2006; 12: 685–718. [DOI] [PubMed] [Google Scholar]
- 3.Ireland JJ, Smith GW, Scheetz D, Jimenez-Krassel F, Folger JK, Ireland JL, Mossa F, Lonergan P, Evans AC. Does size matter in females? An overview of the impact of the high variation in the ovarian reserve on ovarian function and fertility, utility of anti-Müllerian hormone as a diagnostic marker for fertility and causes of variation in the ovarian reserve in cattle. Reprod Fertil Dev 2011; 23: 1–14. [DOI] [PubMed] [Google Scholar]
- 4.Ireland JLH, Scheetz D, Jimenez-Krassel F, Themmen APN, Ward F, Lonergan P, Smith GW, Perez GI, Evans AC, Ireland JJ. Antral follicle count reliably predicts number of morphologically healthy oocytes and follicles in ovaries of young adult cattle. Biol Reprod 2008; 79: 1219–1225. [DOI] [PubMed] [Google Scholar]
- 5.Burns DS, Jimenez-Krassel F, Ireland JLH, Knight PG, Ireland JJ. Numbers of antral follicles during follicular waves in cattle: evidence for high variation among animals, very high repeatability in individuals, and an inverse association with serum follicle-stimulating hormone concentrations. Biol Reprod 2005; 73: 54–62. [DOI] [PubMed] [Google Scholar]
- 6.Mossa F, Walsh SW, Butler ST, Berry DP, Carter F, Lonergan P, Smith GW, Ireland JJ, Evans AC. Low numbers of ovarian follicles ≥3 mm in diameter are associated with low fertility in dairy cows. J Dairy Sci 2012; 95: 2355–2361. [DOI] [PubMed] [Google Scholar]
- 7.Ireland JJ, Ward F, Jimenez-Krassel F, Ireland JLH, Smith GW, Lonergan P, Evans AC. Follicle numbers are highly repeatable within individual animals but are inversely correlated with FSH concentrations and the proportion of good-quality embryos after ovarian stimulation in cattle. Hum Reprod 2007; 22: 1687–1695. [DOI] [PubMed] [Google Scholar]
- 8.Nagai K, Yanagawa Y, Katagiri S, Nagano M. Fertilizability of oocytes derived from Holstein cows having different antral follicle counts in ovaries. Anim Reprod Sci 2015; 163: 172–178. [DOI] [PubMed] [Google Scholar]
- 9.Nagai K, Yanagawa Y, Katagiri S, Nagano M. The relationship between antral follicle count in a bovine ovary and developmental competence of in vitro-grown oocytes derived from early antral follicles. Biomed Res 2016; 37: 63–71. [DOI] [PubMed] [Google Scholar]
- 10.Hirao Y, Itoh T, Shimizu M, Iga K, Aoyagi K, Kobayashi M, Kacchi M, Hoshi H, Takenouchi N. In vitro growth and development of bovine oocyte-granulosa cell complexes on the flat substratum: effects of high polyvinylpyrrolidone concentration in culture medium. Biol Reprod 2004; 70: 83–91. [DOI] [PubMed] [Google Scholar]
- 11.Huang W, Kang SS, Nagai K, Yanagawa Y, Takahashi Y, Nagano M. Mitochondrial activity during pre-maturational culture in in vitro-grown bovine oocytes is related to maturational and developmental competences. Reprod Fertil Dev 2016; 28: 349–356. [DOI] [PubMed] [Google Scholar]
- 12.Yang Y, Kanno C, Sakaguchi K, Yanagawa Y, Katagiri S, Nagano M. Extension of the culture period for the in vitro growth of bovine oocytes in the presence of bone morphogenetic protein-4 increases oocyte diameter, but impairs subsequent developmental competence. Anim Sci J 2017; 88: 1686–1691. [DOI] [PubMed] [Google Scholar]
- 13.Kruip TA, Dieleman SJ. Steroid hormone concentrations in the fluid of bovine follicles relative to size, quality and stage of the oestrus cycle. Theriogenology 1985; 24: 395–408. [DOI] [PubMed] [Google Scholar]
- 14.Sakaguchi K, Huang W, Yang Y, Yanagawa Y, Nagano M. Relationship between in vitro growth of bovine oocytes and steroidogenesis of granulosa cells cultured in medium supplemented with bone morphogenetic protein-4 and follicle stimulating hormone. Theriogenology 2017; 97: 113–123. [DOI] [PubMed] [Google Scholar]
- 15.Harada M, Miyano T, Matsumura K, Osaki S, Miyake M, Kato S. Bovine oocytes from early antral follicles grow to meiotic competence in vitro: effect of FSH and hypoxanthine. Theriogenology 1997; 48: 743–755. [DOI] [PubMed] [Google Scholar]
- 16.Endo M, Kawahara-Miki R, Cao F, Kimura K, Kuwayama T, Monji Y, Iwata H. Estradiol supports in vitro development of bovine early antral follicles. Reproduction 2013; 145: 85–96. [DOI] [PubMed] [Google Scholar]
- 17.Yanagawa Y, Matsuura Y, Suzuki M, Saga S, Okuyama H, Fukui D, Bando G, Nagano M, Katagiri S, Takahashi Y, Tsubota T. Accessory corpora lutea formation in pregnant Hokkaido sika deer (Cervus nippon yesoensis) investigated by examination of ovarian dynamics and steroid hormone concentrations. J Reprod Dev 2015; 61: 61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Y, Kanno C, Huang W, Kang SS, Yanagawa Y, Nagano M. Effect of bone morphogenetic protein-4 on in vitro growth, steroidogenesis and subsequent developmental competence of the oocyte-granulosa cell complex derived from bovine early antral follicles. Reprod Biol Endocrinol 2016; 14: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagano M, Kang SS, Koyama K, Huang W, Yanagawa Y, Takahashi Y. In vitro maturation system for individual culture of bovine oocytes using micro-volume multi-well plate. Jpn J Vet Res 2013; 61: 149–154. [PubMed] [Google Scholar]
- 20.Nagano M, Katagiri S, Takahashi Y. Relationship between bovine oocyte morphology and in vitro developmental potential. Zygote 2006; 14: 53–61. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi Y, Kanagawa H. Effects of glutamine, glycine and taurine on the development of in vitro fertilized bovine zygotes in a chemically defined medium. J Vet Med Sci 1998; 60: 433–437. [DOI] [PubMed] [Google Scholar]
- 22.Brackett BG, Oliphant G. Capacitation of rabbit spermatozoa in vitro. Biol Reprod 1975; 12: 260–274. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi Y, First NL. In vitro development of bovine one-cell embryos: Influence of glucose, lactate, pyruvate, amino acids and vitamins. Theriogenology 1992; 37: 963–978. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi Y, Hishinuma M, Matsui M, Tanaka H, Kanagawa H. Development of in vitro matured/fertilized bovine embryos in a chemically defined medium: influence of oxygen concentration in the gas atmosphere. J Vet Med Sci 1996; 58: 897–902. [DOI] [PubMed] [Google Scholar]
- 25.Taketsuru H, Hirao Y, Takenouchi N, Iga K, Miyano T. Effect of androstenedione on the growth and meiotic competence of bovine oocytes from early antral follicles. Zygote 2012; 20: 407–415. [DOI] [PubMed] [Google Scholar]
- 26.O’Shea JD, Rodgers RJ, D’Occhio MJ. Cellular composition of the cyclic corpus luteum of the cow. J Reprod Fertil 1989; 85: 483–487. [DOI] [PubMed] [Google Scholar]
- 27.Meidan R, Girsh E, Blum O, Aberdam E. In vitro differentiation of bovine theca and granulosa cells into small and large luteal-like cells: morphological and functional characteristics. Biol Reprod 1990; 43: 913–921. [DOI] [PubMed] [Google Scholar]
- 28.Scheetz D, Folger JK, Smith GW, Ireland JJ. Granulosa cells are refractory to FSH action in individuals with a low antral follicle count. Reprod Fertil Dev 2012; 24: 327–336. [DOI] [PubMed] [Google Scholar]
- 29.Abdel-Ghani MA, Sakaguchi K, Kanno C, Yanagawa Y, Katagiri S, Nagano M. Effects of pre-maturational culture duration on developmental competence of bovine small-sized oocytes. J Reprod Dev 2018; 64: 365–369. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Orly J, Sato G, Erickson GF. Serum suppresses the expression of hormonally induced functions in cultured granulosa cells. Cell 1980; 20: 817–827. [DOI] [PubMed] [Google Scholar]
- 31.Kayani AR, Glister C, Knight PG. Evidence for an inhibitory role of bone morphogenetic protein(s) in the follicular-luteal transition in cattle. Reproduction 2009; 137: 67–78. [DOI] [PubMed] [Google Scholar]
- 32.Senbon S, Miyano T. Bovine oocytes in early antral follicles grow in serum-free media: effect of hypoxanthine on follicular morphology and oocyte growth. Zygote 2002; 10: 301–309. [DOI] [PubMed] [Google Scholar]
- 33.Senbon S, Fukumi Y, Hamawaki A, Yoshikawa M, Miyano T. Bovine oocytes grown in serum-free medium acquire fertilization competence. J Reprod Dev 2004; 50: 541–547. [DOI] [PubMed] [Google Scholar]