By pooling SMART/START data, we found that a randomized strategy of interrupted/deferred antiretroviral therapy increases the hazard of AIDS by 3.6, serious non-AIDS (SNA) by 1.6, and the composite of AIDS, SNA, or death by 2.1.
Keywords: antiretroviral therapy, AIDS, cardiovascular disease, cancer, HIV
Abstract
Background
Pooled data from the SMART and START trials were used to compare deferred/intermittent versus immediate/continuous antiretroviral therapy (ART) on disease risk.
Methods
Endpoints assessed were AIDS, serious non-AIDS (SNA), cardiovascular disease (CVD), cancer, and death. Pooled (stratified by study) hazard ratios (HRs) from Cox models were obtained for deferred/intermittent ART versus immediate/continuous ART; analyses were conducted to assess consistency of HRs across baseline-defined subgroups.
Results
Among 10156 participants, there were 124 AIDS, 247 SNA, 117 cancers, 103 CVD, and 120 deaths. Interventions in each trial led to similar differences in CD4 count and viral suppression. Pooled HRs (95% confidence interval) of deferred/intermittent ART versus immediate/continuous ART were for AIDS 3.63 (2.37–5.56); SNA 1.62 (1.25–2.09); CVD 1.59 (1.07–2.37); cancer 1.93 (1.32–2.83); and death 1.80 (1.24–2.61). Underlying risk was greater in SMART than START. Given the similar HRs for each trial, absolute risk differences between treatment groups were greater in SMART than START. Pooled HRs were similar across subgroups.
Conclusions
Treatment group differences in CD4 count and viral suppression were similar in SMART and START. Likely as a consequence, relative differences in risk of AIDS and SNA between immediate/continuous ART and deferred/intermittent ART were similar.
Clinical Trials Registration
NCT00027352 and NCT00867048.
(See the Editorial Commentary by Siedner and Triant on pages173–6.)
The Strategies for Management of Antiretroviral Therapy (SMART) [1] and Strategic Timing of AntiRetroviral Treatment (START) [2] studies were the seminal trials to assess the effect of antiretroviral therapy (ART) treatment strategies on risk of both AIDS- and non-AIDS–defining events. These 2 studies, together with other trials [3, 4], established continuous/immediate ART as the standard of care for human immunodeficiency virus (HIV)-positive persons.
SMART and START were halted prematurely, by the respective data safety monitoring boards for each trial, before the target numbers of primary endpoints were reached. Therefore, each trial had a limited ability to quantify variation in risk of some outcomes such as cardiovascular disease (CVD) and cancer. To address this, we conducted a pooled analysis of the 2 trials in which we compared the drug conservation arm [DC] in SMART and the deferred ART arm in START (the “deferred/intermittent ART group”) with the viral suppression arm [VS] in SMART and the immediate ART arm in START (the “immediate/continuous ART group”). We hypothesized that treatment hazard ratios would be similar in each study and that the pooled analysis would better quantify the relative difference between deferred/intermittent ART and immediate/continuous ART on risk of AIDS- and non-AIDS–defining events.
METHODS
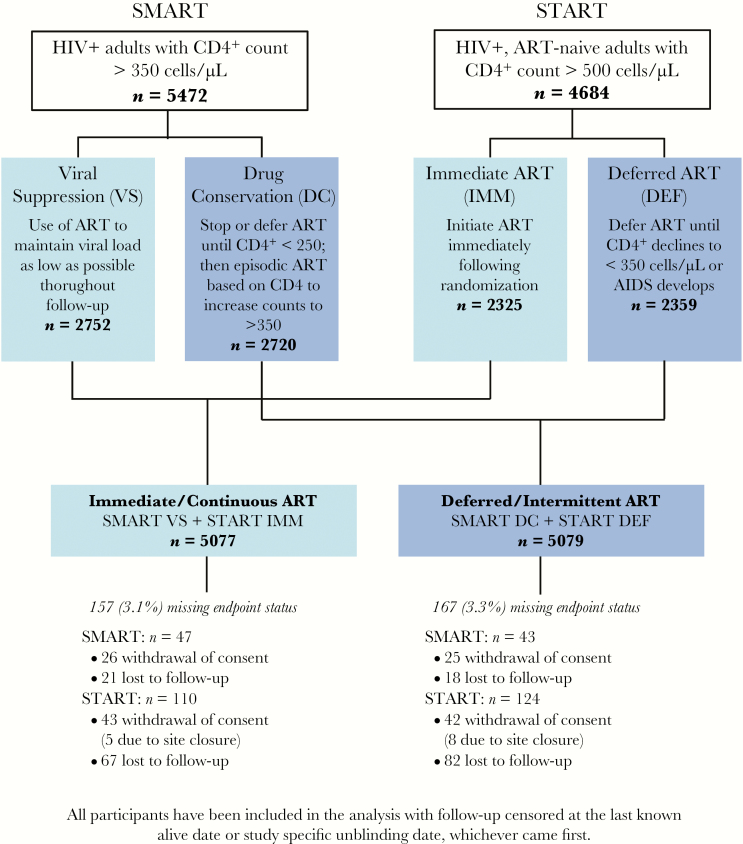
SMART (NCT00027352) was a randomized controlled trial of 5472 HIV-positive persons with CD4+ >350 cells/µL at baseline that compared continuous use of ART VS with structured treatment interruptions until the CD4+ cell count declined to less than 250 cells/µL DC [1] (Figure 1). Recruitment occurred from January 2002 to January 2006, and the study was unblinded in January 2006 before the planned enrolment was achieved. START (NCT00867048) was a randomized trial of 4684 HIV-positive ART-naive persons with CD4+ >500 cells/µL at baseline that compared immediate ART initiation (immediate ART arm [IMM]) with deferred ART initiation until CD4+ cell counts dropped below 350 cells/µL or development of AIDS (deferred ART arm [DEF]) [2] (Figure 1). The study enrolled from April 2009 to December 2013 and was unblinded in May 2015.
Figure 1.
Study design. Abbreviations: ART, antiretroviral therapy; DC, drug conservation; DEF, deferred ART; HIV, human immunodeficiency virus; IMM, immediate; ART; SMART, Strategies for Management of Antiretroviral Therapy; START, Strategic Timing of Antiretroviral Treatment; VS, viral suppression.
SMART and START were conducted in compliance with the Declaration of Helsinki Guidelines, were registered on clinical trial databases, and reviewed by independent data and safety interim monitoring boards. An institutional review board/ethics committee at each study site approved the study protocol, and written informed consent was obtained from all participants.
A design schematic for the comparison of the “deferred/intermittent ART group” with the “immediate/continuous ART group” is illustrated in Figure 1. Follow-up was censored at the last known alive date or study unblinding (11 January 2006 for SMART and 25 May 2015 for START), whichever occurred first. Definitions of AIDS and serious non-AIDS (SNA) were harmonized in SMART and START to match those in START [2, 5]. Individual events comprising these composite outcomes were defined similarly [6–8]. The adjudicated clinical outcomes considered in this report were: (1) AIDS or AIDS death; (2) SNA including CVD, non-AIDS cancer, end-stage renal disease, decompensated liver disease, and non-AIDS death; (3) CVD including myocardial infarction, stroke, coronary revascularization, and CVD death; (4) AIDS and non-AIDS, infection-related and -unrelated cancer, and cancer death; and (5) all-cause death. Causes of death were coded using the Coding Causes of Death (CoDe) system [9]. Cancer classification as infection-related or infection-unrelated was on the basis of individual review of case report forms and source documentation. Infection-related cancer was defined as cancer driven by the following infectious agents: human herpesvirus 8 (Kaposi sarcoma), Epstein-Barr virus (non-Hodgkin lymphoma, Hodgkin lymphoma), and human papilloma virus (anal cancer, cervical cancer) [10–12]. All other malignancies were classified as infection-unrelated cancer.
Hazards ratios (HRs) for deferred/intermittent ART versus immediate/continuous ART were obtained from Cox models with a single indicator for treatment group for each trial separately. Pooled HRs were estimated from Cox models, stratified by study (SMART vs START), that included a single indicator variable for deferred/intermittent ART versus immediate/continuous ART. Heterogeneity of HRs between SMART and START were assessed from models that included an interaction term between the pooled treatment group (deferred/intermittent ART vs immediate/continuous ART) and an indicator for study. HRs for deferred/intermittent ART versus immediate/continuous ART were estimated for each cancer grouping (infection-related or infection-unrelated) in a Cox model that considered multiple events per participant [13]. To assess consistency of results across baseline-defined subgroups defined by age, gender, race, CD4+ count, CD4:CD8 ratio, income region, smoking status, body mass index, hepatitis B/C coinfection, d-dimer, and interleukin-6 (IL-6) levels, expanded Cox models that included interaction terms for the baseline subgrouping variable and the pooled treatment indicator were used.
In the analyses that compare HRs for SMART and START and in the subgroup analyses of the pooled HRs by baseline subgroups, the interaction P values should be viewed cautiously. Multiple endpoints are considered in the comparisons of SMART with START and multiple baseline subgroups were considered for the pooled HR. This increases the risk of a type 1 error. Also, the interaction test lacks power, and for many outcomes the number of events is small so there is a risk of a type 2 error.
Changes in CD4+ cell count from baseline through follow-up between the deferred/intermittent ART and immediate/continuous ART groups (within each trial and pooled) were compared using longitudinal mixed models with random intercepts. Viral suppression, defined as HIV RNA ≤400 copies/mL, was plotted over time, and the percent of follow-up time with viral suppression was estimated.
Analyses were performed with SAS, version 9.3 (SAS Institute, Cary, NC). P values are 2-sided and values ≤.05 were considered significant. No adjustments were made for multiple comparisons.
RESULTS
The total number of participants was 10156, comprising 4684 from START and 5472 from SMART (median age 40 years; 27% female; 51% men who have sex with men; median baseline CD4+ 634 cells/µL; 37% smokers) (Table 1). Except for gender distribution, SMART and START participants differed markedly in terms of demographics, HIV-specific variables, hepatitis coinfection, smoking, and ART (Table 1). Median nadir CD4+ count was 250 cells/µL in SMART and 553 cells/µL in START. SMART participants were also older and the majority (84%) were receiving ART at baseline (Table 1). Median (interquartile range) follow-up was 1.0 (0.4–2.1) years in SMART and 3.0 (2.2–4.1) years in START.
Table 1.
Baseline Characteristics
| Characteristic | START (n = 4684) | SMART (n = 5472) | P Valuea (START vs SMART) | Overall (n = 10 156) |
|---|---|---|---|---|
| Median age, years (IQR) | 36 (29–44) | 43 (38–50) | <.001 | 40 (33–48) |
| Female (%) | 26.8 | 27.2 | .72 | 27.0 |
| Race or ethnic group (%) | ||||
| Black | 30.1 | 29.1 | 29.6 | |
| Latino or Hispanic | 13.6 | 21.1 | 17.6 | |
| Asian | 8.3 | 4.1 | 6.0 | |
| White | 44.6 | 43.6 | 44.0 | |
| Other | 3.5 | 2.1 | 2.8 | |
| Geographical region (%) | ||||
| Africa | 21.3 | 1.2 | 10.5 | |
| Asia | 7.6 | 3.2 | 5.2 | |
| Australia | 2.3 | 3.2 | 2.8 | |
| Europe | 32.9 | 25.8 | 29.1 | |
| North America | 10.8 | 56.5 | 35.4 | |
| Latin America | 25.1 | 10.0 | 17.0 | |
| % from high income country | 46.0 | 85.6 | 67.3 | |
| Median years since HIV diagnosis (IQR) | 1 (0–3) | 8 (5–12) | <.001 | 4 (1–9) |
| Median CD4, cells/µL (IQR) | 651 (584–765) | 597 (466–790) | <.001 | 634 (539–776) |
| Median CD4:CD8 ratio (IQR)b | 0.66 (0.48–0.89) | 0.69 (0.48–0.97) | <.001 | 0.67 (0.48–0.93) |
| Median nadir CD4, cells/µL (IQR) | 553 (488–654) | 250 (154–358) | <.001 | 418 (237–558) |
| Median HIV RNA, copies/mL (IQR)b | 12 761 (3025–43 482) | 80 (50–793) | <.001 | 1568 (50–19 274) |
| HIV RNA ≤400 copies/mL (%) | 7.9 | 71.7 | 42.3 | |
| On ART at baseline (%) | 0.0 | 84.0 | 45.2 | |
| ART naive (%) | 100.0 | 4.6 | 48.6 | |
| Hepatitis coinfection (%)b | 6.4 | 17.0 | <.001 | 12.2 |
| Current smoker (%) | 32.0 | 40.5 | <.001 | 36.6 |
| Median BMI, kg/m2 (IQR)b | 24.6 (22.1–27.9) | 24.9 (22.4–28.1) | <.001 | 24.8 (22.3–28.0) |
| Median IL-6, pg/mL (IQR)b | 1.39 (0.97–2.12) | 1.77 (1.09–3.01) | <.001 | 1.57 (1.03–2.57) |
| Median d-dimer, µg/mL (IQR)b | 0.33 (0.23–0.50) | 0.22 (0.13–0.38) | <.001 | 0.27 (0.17–0.45) |
Abbreviations; ART, antiretroviral therapy; BMI, body mass index; IL-6, interleukin-6; IQR, interquartile range; SMART, Strategies for Management of Antiretroviral Therapy; START, Strategic Timing of Antiretroviral Treatment.
a P value comparing SMART and START for selected baseline characteristics that did not reflect differences due to study design/conduct. Χ2 test for categorical variables and Wilcoxon rank sum test for continuous variables.
bMissing baseline data CD4:CD8 ratio: START = 56, SMART = 2637; HIV RNA: START = 9, SMART = 15; hepatitis B/C coinfection: START = 54, SMART = 8; BMI: SMART = 21; IL-6: START = 388, SMART = 435; d-dimer: START = 410, SMART = 403.
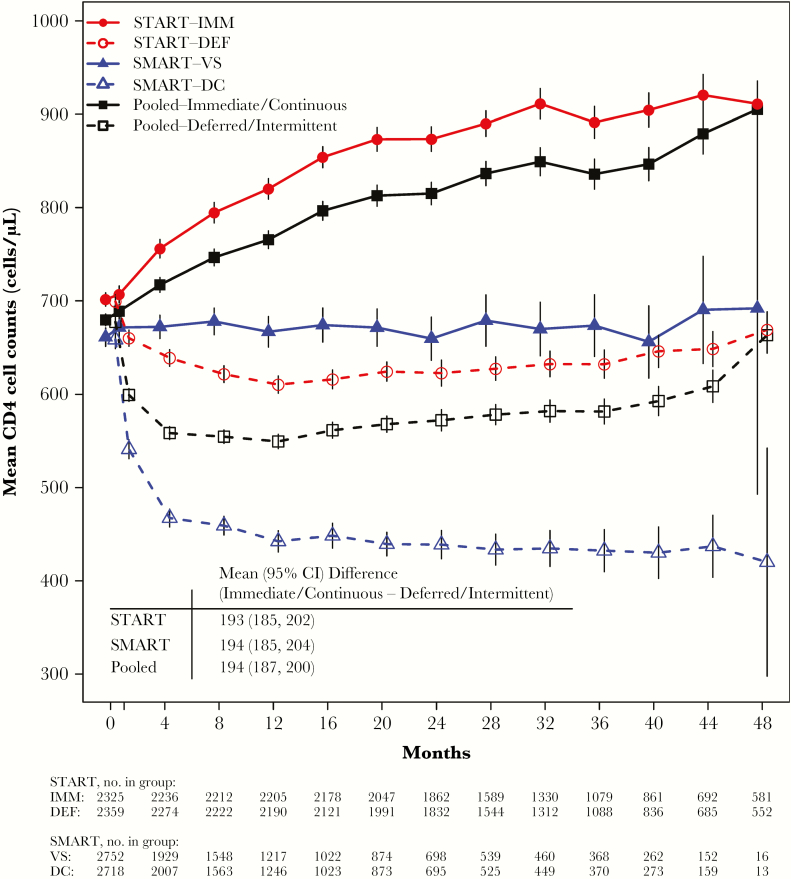
During follow-up, the mean CD4+ cell count was 194 (95% confidence interval [CI], 187–200) cells/µL higher in the immediate/continuous ART group than the deferred/intermittent ART group (Figure 2). The average CD4+ count difference between treatment groups in SMART and START were almost identical; 194 vs 193 cells/µL. The treatment differences in CD4+ counts between deferred/intermittent ART group and immediate/continuous ART group became clear 8 months after randomization and remained so throughout follow-up (Figure 2).
Figure 2.
Mean CD4 cell counts over follow-up by treatment group for START, SMART, and the pooled deferred/intermittent and immediate/continuous ART groups. The vertical lines around the data points represent the 95% confidence interval (CI). Graph is truncated at 48 months. The table within the figure presents the mean CD4 cell count difference (with 95% CI) over follow-up between the immediate and deferred groups in START, the viral suppression and drug conservation groups in SMART, and the pooled immediate/continuous and deferred/intermittent ART groups. Abbreviations: ART, antiretroviral therapy; DC, drug conservation; DEF, deferred ART; IMM, immediate ART; SMART, Strategies for Management of Antiretroviral Therapy; START, Strategic Timing of Antiretroviral Treatment; VS, viral suppression.
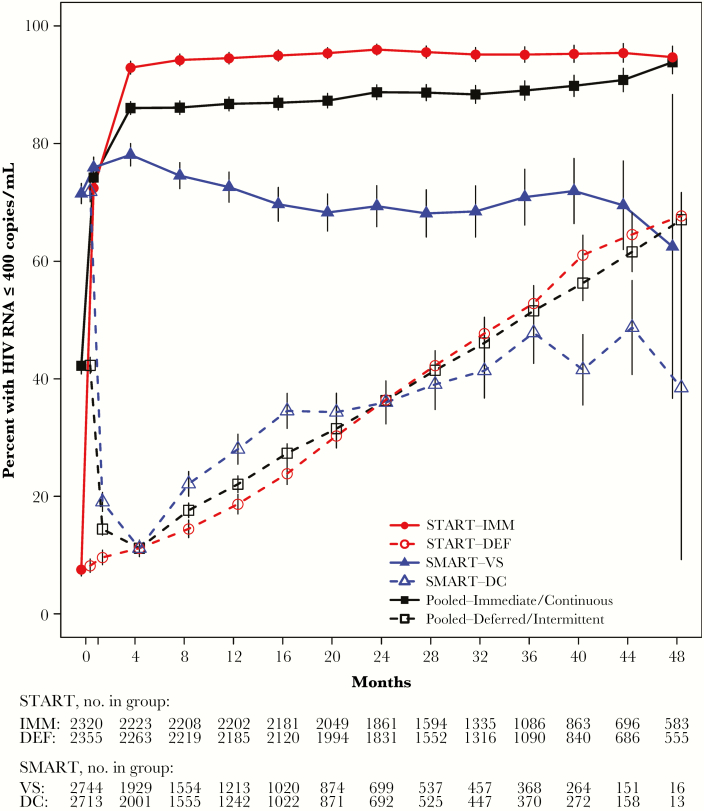
Compared to the deferred/intermittent ART group, the immediate/continuous ART group had a greater proportion of participants with an HIV RNA level ≤400 copies/mL for more of the follow-up period (Figure 3). The percent of follow-up time spent with a viral load ≤400 copies/mL was 84.2% in the immediate/continuous ART group compared to 33.3% in the deferred/intermittent ART group. Similar differences were seen in each study (30.6% vs 89.2% in the DEF vs IMM arms of START; 38.7% vs 74.1% in the DC vs VS arms of SMART).
Figure 3.
Percent of participants with human immunodeficiency virus (HIV) RNA ≤400 copies/mL over follow-up, by treatment group for START, SMART, and the pooled deferred/intermittent and immediate/continuous ART groups. Graph is truncated at 48 months. Abbreviations: ART, antiretroviral therapy; DC, drug conservation; DEF, deferred ART; IMM, immediate ART; SMART, Strategies for Management of Antiretroviral Therapy; START, Strategic Timing of Antiretroviral Treatment; VS, viral suppression.
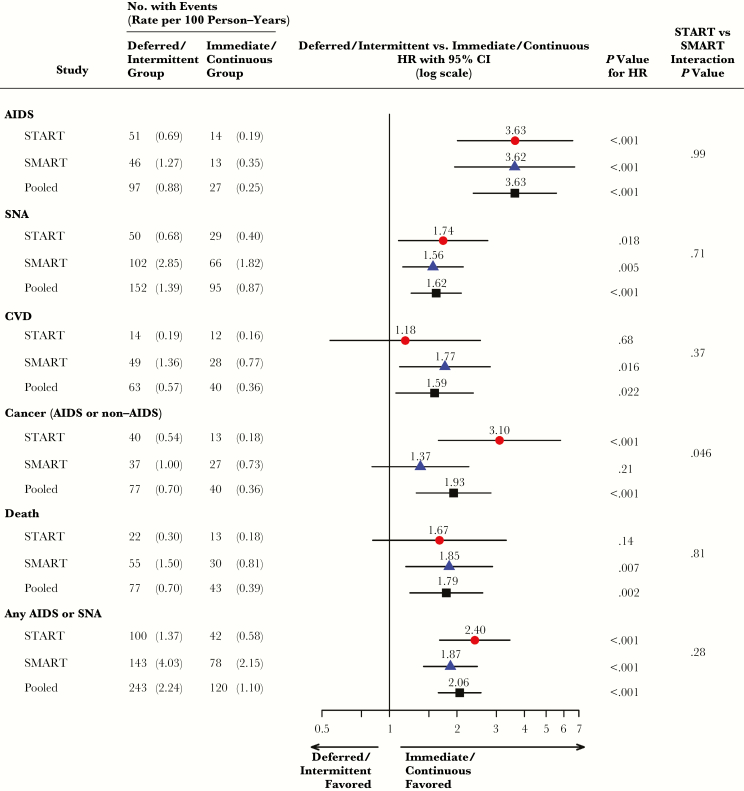
During 22103 person-years of follow-up, there were 124 AIDS events (fatal or nonfatal), 247 SNA or non-AIDS deaths, and 363 AIDS, SNA, or death events. Pooled HRs of deferred/intermittent ART group versus immediate/continuous ART group were 3.63 (95% CI, 2.37–5.56) for AIDS, 1.62 (95% CI, 1.25–2.09) for SNA, and 2.06 (95% CI, 1.65–2.56) for the composite of AIDS, SNA, or death (Figure 4). The START and SMART HRs for these outcomes were very similar; 3.63 and 3.62 for AIDS (P = .99 for interaction), 1.74 and 1.56 for SNA (P = .71), and 2.40 and 1.87 (P = .28) for the composite of AIDS, SNA, or death.
Figure 4.
Hazard ratios (HRs) with 95% confidence intervals (95% CI) comparing event risk by treatment group within START and SMART separately, and between the pooled deferred/intermittent and immediate/continuous ART groups. The P value for interaction is comparing the HR for each event between the deferred versus immediate group within START to the HR for the same event between the viral suppression versus drug conservation group within SMART. Abbreviations: CVD, cardiovascular disease; SMART, Strategies for Management of Antiretroviral Therapy; SNA, serious non-AIDS; START, Strategic Timing of Antiretroviral Treatment.
For both AIDS and SNA, event rates were much higher in SMART than START. Coupled with the similar HRs, that resulted in absolute risk differences much larger for SMART than START (0.92 versus 0.50 per 100 person years for AIDS and 1.03 versus 0.28 per 100 person years for SNA). There is greater heterogeneity between the SMART and START HRs for CVD and cancer (P values for interaction are 0.37 and 0.046, respectively). In both trials, the pooled HRs are significantly greater than 1.0: 1.59 (95% CI, 1.07–2.37) for CVD and 1.93 (95% CI, 1.32–2.83) for cancer. The pooled HR for death was 1.79 (95% CI, 1.24–2.61).
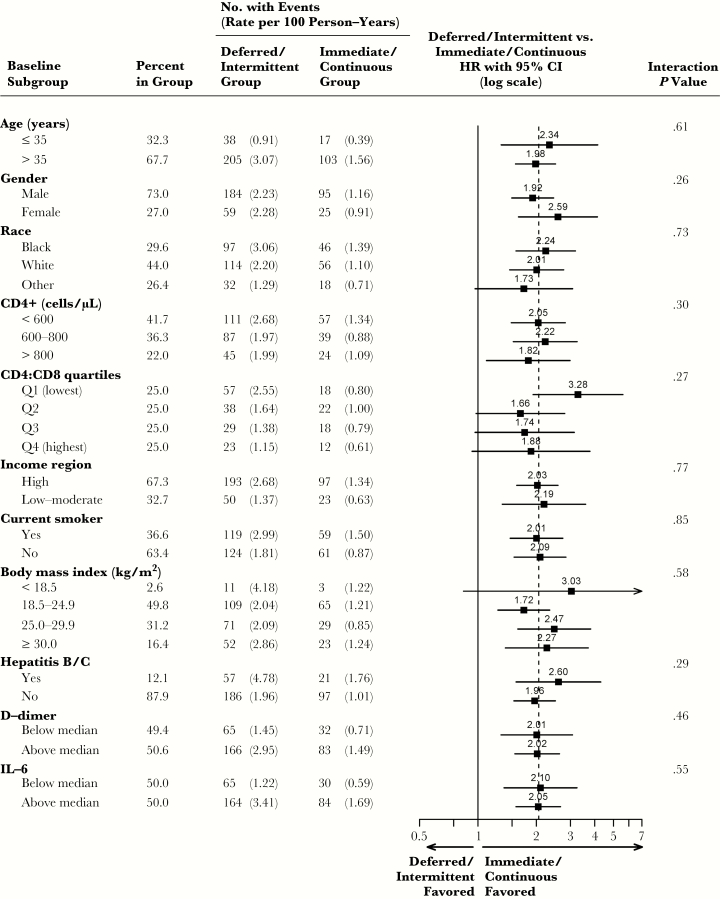
The HR of deferred/intermittent ART group versus immediate/continuous ART group for infection-related cancer was 2.3 (95% CI, 1.3–4.3), P = .006, compared to 1.7 (95% CI, 1.0–2.8), P = .04, for infection-unrelated cancer. P value comparing the HRs for infection-related versus infection-unrelated cancer was .43. Deferred/intermittent ART was consistently associated with increased risk of the composite outcome of AIDS, SNA, or death events across all subgroups investigated (interaction p>0.25, Figure 5).
Figure 5.
Subgroup analyses for the composite endpoint of AIDS, SNA, or death events; hazard ratios (HRs) with 95% confidence intervals (95% CI) comparing the deferred/intermittent ART group versus immediate/continuous ART group within the pooled cohort. For subgroups of age, CD4 cell count, CD4:CD8 ratio, body mass index, d-dimer, and IL-6, the continuous variables were used for the interaction tests. Baseline CD4:CD8 ratio, hepatitis B/C coinfection, d-dimer, and IL-6 were not available for all participants; HRs within subgroups for these measures were computed using participants with available baseline data. Abbreviations: ART, antiretroviral therapy; IL-6, interleukin-6; SNA, serious non-AIDS.
DISCUSSION
We pooled data from 2 landmark global HIV trials and found that a randomized strategy, consisting of deferred/intermittent ART, increases the hazard of AIDS by 3.6, SNA by 1.6, and for the composite outcome of AIDS, SNA, or death by 2.1 compared to immediate/continuous ART. Pooled treatment differences were similar across the subgroups investigated. The present study is the best randomized evidence that a strategy of deferred/intermittent ART leads to an increased risk of AIDS-defining and SNA–defining conditions.
Compared with START participants, SMART participants were older, had lower nadir CD4+ counts, lower follow-up CD4+ counts, and had been diagnosed with HIV for a much longer period of time. As a consequence of these and other, as yet unknown, differences, event rates were much higher in SMART as compared to START. The similar relative differences in risk for the 2 studies coupled with the higher event rate in SMART resulted in much greater absolute risk differences in SMART compared to SMART.
Our findings indicate that the relative vulnerability to develop immunodeficiency-related phenotypes is not affected by the degree of prior immunodeficiency. It appears that the relative effect of ART on disease risk is determined by the same factors irrespective of when ART is initiated. Interventions in SMART and START led to similar differences in CD4+ cell count and viral suppression, and these differences likely led to the similar HRs for the 2 studies. With respect to CD4+ cell counts, shifting the distribution from a mean of about 650 to 450 cells/µL during follow-up had the same impact on relative risk of AIDS and SNA as shifting it from 850 to 650 cells/µL (Figure 2).
Differences between SMART and START regarding cardiovascular outcomes have attracted interest from HIV researchers [14, 15]. ART interruptions in SMART led to a 70% increased CVD risk [1]; whereas in START no significant differences in CVD risk [2] or surrogate markers of atherosclerosis [16, 17] were observed. Hunt et al hypothesized that the much lower nadir CD4+ counts of SMART participants, resulting from later ART initiation, were associated with a deeper perturbation of inflammatory-related pathways which, in turn, could drive a spectrum of end-organ diseases and cancers differently from that observed among START participants with higher nadir CD4+ counts [14]. It is also possible, as the P value for interaction suggests, that the difference in HRs between the 2 trials for CVD is due to chance.
We have observed that the increased CVD risk for the deferred/intermittent ART group compared to the immediate/continuous ART group was consistent, without evidence of heterogeneity between SMART and START, despite a difference in nadir CD4+ counts of 300 cells/µL. However, observed HRs for CVD were numerically different in each trial (1.18 for START versus 1.77 in SMART). Lower HRs for START may have reflected the small number of CVD events and insufficient power to detect significant differences in risk. Follow-up was short in START and participants had an inherent low cardiovascular risk at study entry. Efforts are underway to extend follow-up among START participants until 2021. This will allow us to determine with accuracy HRs for CVD events in the future. Higher levels of inflammation and coagulation markers measured at study entry were significantly associated with subsequent CVD risk both among SMART [18] and START participants [17]. The definition of CVD, as a part of the composite endpoint for each trial, was virtually the same. Hence, we believe that the variation in absolute CVD risk between SMART and START likely reflects differences in prevalent risk factors between the 2 study populations. For instance, SMART participants were older, had a higher prevalence of traditional CVD risk factors, and received more toxic and old-fashioned ART regimens than START participants.
The impact of continuous ART in SMART [19] and immediate ART in START [12] in reducing the risk of infection-related cancer was significant and comparable between the 2 studies. As for infection-unrelated cancer, it remained unclear whether the nonsignificant reduction in risk in both studies [12, 19] reflected limited power. The present pooled analysis combining START and SMART had enough statistical power to better estimate the impact of ART strategies on the risk of infection-unrelated cancer than each study separately. The significant increase in risk associated with deferred/intermittent ART did not vary by type of cancer, and was consistently observed for infection-related and -unrelated cancer (P = .43).
In a previous report, we showed that the benefit of ART in reducing cancer risk was not entirely attributable to HIV RNA suppression [12]. Possible mediators of ART benefit could have been a curb on oncogenic virus coinfection and/or reduction of inflammation [12, 20]. Enhanced inflammation and coagulation, as demonstrated by higher levels of IL-6 and d-dimer, were associated with higher risk of cancer (both infection-related and -unrelated) among SMART participants [11]. In START, however, these biomarkers did not predict cancer risk [18]. The interplay between ART, inflammation, and cancer risk is therefore complex; further research is needed to identify mediators of the benefit of immediate/continuous ART in reducing cancer risk.
Our study has limitations to be acknowledged. First, SMART and START were distinct clinical experiments testing different ART strategies in study populations with dissimilar underlying risk profiles. Still, a pooled analysis of SMART and START seemed to us justified given the similar outcomes in deferred/intermittent ART versus immediate/continuous ART (Figure 4) and the larger number of individual endpoints for a better risk estimation. Second, the number of rare but very clinically relevant outcomes, such as end-stage renal and liver diseases, was too small to allow risk estimation. As a result, these heterogeneous outcomes had to be combined as SNA. With respect to cancer and CVD, however, our analyses had more power. Third, START recruited participants from low-resource settings with limited diagnostic capacity. It is possible that some outcomes, such as myocardial infarction, might have been underdiagnosed. Fourth, we did not present pooled data on ART-related complications because events were collected differently in each trial. In SMART, there were more grade 4 events in the DC than VS arm (173 vs 148; P = .13). Similarly, there were more events for a composite of grade 4 or death in DC than VS (205 vs 164; P = .03) [1]. In START, we collected and reported grade 4 events and unscheduled hospitalizations as separate items [2, 5]. Like SMART, there were fewer of these events in the immediate ART arm. For the composite of grade 4, unscheduled hospitalization or death there were 283 participants with at least 1 event in the immediate group and 311 in the deferred group (P = .25). On the basis of these results, one can infer that no increased toxicity should be expected from immediate/continuous ART compared to deferred/intermittent ART.
To conclude, compared to a strategy of immediate/continuous ART, a strategy of deferred/intermittent ART, increased the risk of AIDS and SNA events among HIV-positive persons consistently in SMART and START. Interventions in SMART and START led to similar absolute differences in mean CD4+ cell count and percentage with viral suppression, which in turn led to similar relative risk estimates for AIDS and SNA in each study. Pooled treatment differences for the composite outcome of AIDS, SNA, or death were similar across a number of subgroups.
Author contributions. A. H. B., J. N., S. S., J. D. N., and J. D. L. conceived the study. J. N. and S. S. performed the statistical analyses. A. H. B. drafted the manuscript. All authors contributed to data interpretation, critically revised the manuscript, and approved the final version.
Acknowledgments. We would like to thank the SMART and START investigators and participants. See N Engl J Med 2006; 355:2283–96 for the complete list of SMART investigators and N Engl J Med 2015; 373:795–807 (supplementary material) for the complete list of START investigators.
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of this manuscript.
Financial support. This work was supported by National Institutes of Health (grant numbers U01AI068641, U01AI042170, and U01AI46362 [SMART]; UM1-AI068641 and UM1-AI120197 [START]). START was supported by the National Institute of Allergy and Infectious Diseases (United States), Agence Nationale de Recherches sur le SIDA et les Hipatites Virales (France), National Health and Medical Research Council (Australia), Danish National Research Foundation (Denmark), Bundes ministerium für Bildung und Forschung (Germany), European AIDS Treatment Network, Medical Research Council (United Kingdom), National Institute for Health Research, National Health Service (United Kingdom), and the University of Minnesota. Antiretroviral drugs were donated to the central drug repository by AbbVie, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline/ViiV Healthcare, Janssen Scientific Affairs, and Merck. The present study was also supported by the Research Council at Rigshospitalet, Danish National Research Foundation (grant number DNRF126), and Lundbeckfonden (grant number R219-2016-762 to A. H. B.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: 24th Conference on Retroviruses and Opportunistic Infections, 13–16 February 2017, Seattle. Abstract 474.
References
- 1. El-Sadr WM, Lundgren J, et al. ; Strategies for Management of Antiretroviral Therapy (SMART) Study Group. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med 2006; 355:2283–96. [DOI] [PubMed] [Google Scholar]
- 2. Lundgren JD, Babiker AG, Gordin F, et al. ; INSIGHT START Study Group Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015; 373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Danel C, Moh R, Gabillard D, et al. ; TEMPRANO ANRS 12136 Study Group A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med 2015; 373:808–22. [DOI] [PubMed] [Google Scholar]
- 4. Cohen MS, Chen YQ, McCauley M, et al. ; HPTN 052 Study Team Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med 2016; 375:830–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Babiker AG, Emery S, Fätkenheuer G, et al. ; INSIGHT START Study Group Considerations in the rationale, design and methods of the Strategic Timing of AntiRetroviral Treatment (START) study. Clin Trials 2013; 10:S5–S36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lifson AR, Rhame FS, Belloso WH, et al. . Reporting and evaluation of HIV-related clinical endpoints in two multicenter international clinical trials. HIV Clin Trials 2006; 7:125–41. [DOI] [PubMed] [Google Scholar]
- 7. Lifson AR, Belloso WH, Carey C, et al. ; INSIGHT Cause of Death Writing Group. Determination of the underlying cause of death in three multicenter international HIV clinical trials. HIV Clin Trials 2008; 9:177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lifson AR, Belloso WH, Davey RT, et al. ; INSIGHT Endpoint Review Committee Writing Group Development of diagnostic criteria for serious non-AIDS events in HIV clinical trials. HIV Clin Trials 2010; 11:205–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kowalska JD, Friis-Møller N, Kirk O, et al. ; CoDe Working Group; D:A:D Study Group The coding causes of death in HIV (CoDe) project: initial results and evaluation of methodology. Epidemiology 2011; 22:516–23. [DOI] [PubMed] [Google Scholar]
- 10. Bouvard V, Baan R, Straif K, et al. ; WHO International Agency for Research on Cancer Monograph Working Group A review of human carcinogens–Part B: biological agents. Lancet Oncol 2009; 10:321–2. [DOI] [PubMed] [Google Scholar]
- 11. Borges ÁH, Silverberg MJ, Wentworth D, et al. ; INSIGHT SMART; ESPRIT; SILCAAT Study Groups Predicting risk of cancer during HIV infection: the role of inflammatory and coagulation biomarkers. AIDS 2013; 27:1433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Borges ÁH, Neuhaus J, Babiker AG, et al. ; INSIGHT START Study Group Immediate antiretroviral therapy reduces risk of infection-related cancer during early HIV infection. Clin Infect Dis 2016; 63:1668–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wei LJ, Lin DY, Weissfeld L. Regression analysis of multivariate incomplete failure time data by modeling marginal distribution. J Am Stat Assoc 1989; 84:1065–73. [Google Scholar]
- 14. Hunt PW, Lee SA, Siedner MJ. Immunologic biomarkers, morbidity, and mortality in treated HIV infection. J Infect Dis 2016; 214(Suppl 2):S44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Siedner MJ. START or SMART? Timing of antiretroviral therapy initiation and cardiovascular risk for people with human immunodeficiency virus infection. Open Forum Infect Dis 2016; 3:ofw032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Baker JV, Sharma S, Achhra AC, et al. . Changes in cardiovascular disease risk factors with immediate versus deferred antiretroviral therapy initiation among HIV-positive participants in the START (Strategic Timing of Antiretroviral Treatment) trial. J Am Heart Assoc 2017; 6:pii: e004987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Baker JV, Hullsiek KH, Engen NW, et al. . Early antiretroviral therapy at high CD4 counts does not improve arterial elasticity: a substudy of the strategic timing of antiretroviral treatment (START) trial. Open Forum Infect Dis 2016; 3:ofw213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baker JV, Sharma S, Grund B, et al. . Systemic inflammation, coagulation, and clinical risk in the START trial. Open Forum Infect Dis 2017; 4:ofx262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Silverberg MJ, Neuhaus J, Bower M, et al. . Risk of cancers during interrupted antiretroviral therapy in the SMART study. AIDS 2007; 21:1957–63. [DOI] [PubMed] [Google Scholar]
- 20. Borges ÁH. Combination antiretroviral therapy and cancer risk. Curr Opin HIV AIDS 2017; 12:12–9. [DOI] [PMC free article] [PubMed] [Google Scholar]