FIGURE 8.
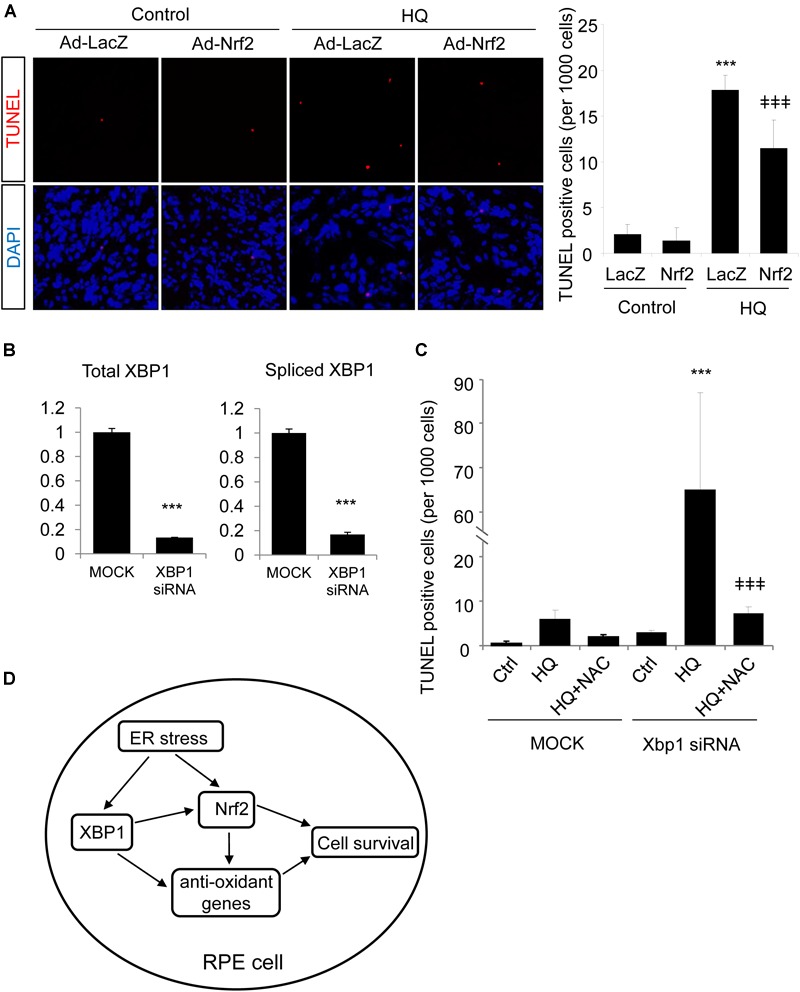
Overexpression of Nrf2 protects ARPE-19 cells from hydroquinone induced injury. (A) ARPE-19 cells were transfected with adenovirus overexpressing Nrf2 or LacZ as control for 24 h, then treated with a potent pro-oxidant, hydroquinone (100 μM, 24 h), for another 24 h. Cell DNA fragmentation was detected by TUNEL assay and quantified by cell counting (mean ± SD, n = 5 random 10× microscope field, ∗∗∗p < 0.001 vs. control, ‡‡‡p < 0.001 vs. Ad-LacZ transfected cells with HQ treatment). (B) ARPE-19 cells were transfected with XBP1 siRNA or lipofectamine only (Mock) as control for 24 h, XBP1 knockdown efficiency was detected by real-time RT PCR (∗∗∗p < 0.001). (C) ARPE-19 cells were transfected with XBP1 siRNA or lipofectamine only (Mock) as control for 24 h, then treated with hydroquinone (100 μM, 24 h) with or without N-Acetylcysteine (NAC) pre-treatment (1 mM, 2 h). Cell DNA fragmentation was detected by TUNEL assay and quantified by cell counting (mean ± SD, n = 5 random 10× microscope field, ∗∗∗p < 0.001 vs. untreated XBP1 siRNA transfected cells, ‡‡‡p < 0.001 vs. XBP1 siRNA transfected and HQ treated cells). (D) Schema of the regulation of Nrf2 by XBP1 in RPE cells. In the RPE, XBP1 upregulates the expression of Nrf2, promotes cell survival against oxidative stress. Loss of XBP1 leads to compromised Nrf2 synthesis, which promotes cell injury induced by oxidants. Meanwhile, XBP1 also regulates other anti-oxidative genes including Catalase, SOD1 and SOD2, which also contributes to anti-oxidative response of the RPE. Overexpression of Nrf2 protects RPE cells from oxidants-induced injury, but could not compensate the loss of XBP1 on cell survival during oxidative stress.
