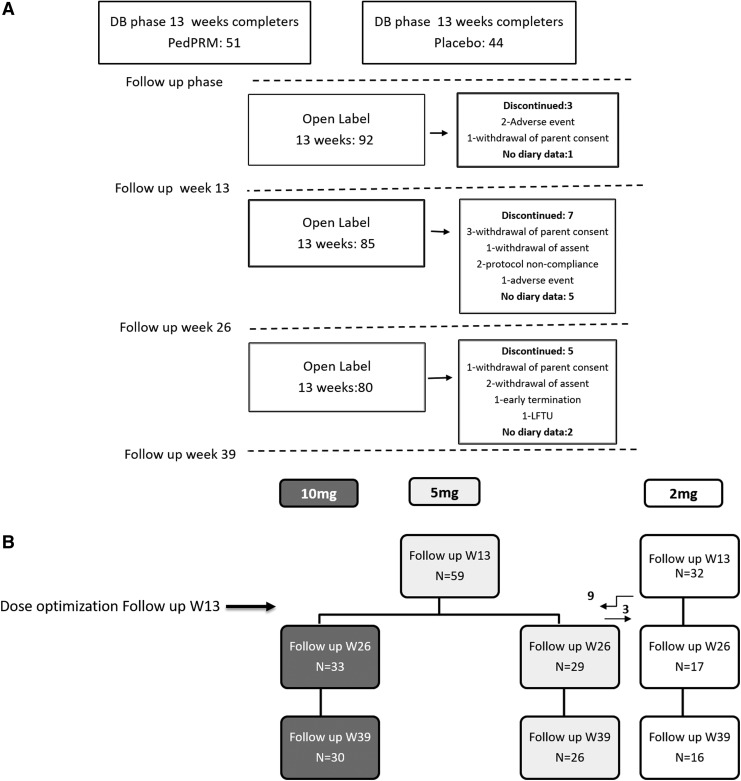
FIG. 1.
Overall study patient disposition (CONSORT diagram) (A) and dose breakdown for patients with SNDs (B). The study comprised 9-month, open-label PedPRM treatment on 2 or 5 mg doses with optional dose adjustment after 13 weeks of the open-label phase. PedPRM, pediatric prolonged-release melatonin; SND, Sleep and Nap Diary.