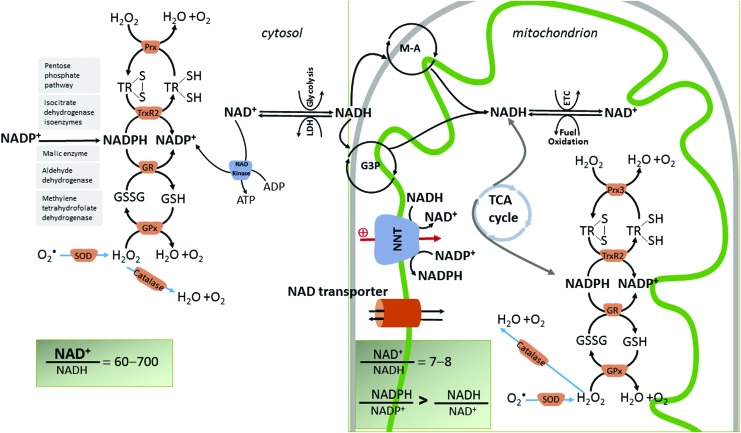
FIG. 3.
The four main redox couples governing the redox balance in cardiac mitochondria (NAD+/NADH, NADP+/NADPH, GSH/GSSG, and TrxSH2/TrxSS) are hinged by NNT. Normal cardiomyocytes maintain a constant NAD pool mostly by converting biosynthetic precursors via the salvage pathway rather than de novo synthesis [not shown in the figure, revised in (83)]. Mitochondrial NAD content is increased by the import of cytosolic NAD through hypothetical transport mechanisms (35) that were identified in many species but not in mammals. However, it is believed that a bi-directional mitochondrial-cytosol transport system exists for both NAD and its precursors because exogenously added NAD leads to a greater accumulation in mitochondria than the cytosol (145). NAD+ is both a coenzyme for redox enzymes and an enzyme substrate for nonredox reactions in which the adenine diphosphate ribose moiety of NAD+ is cleaved, leading to the depletion of the NAD pool (not shown). Nicotinamide adenine nucleotide (NAD+) is a phosphate acceptor being converted to the phosphorylated form, NADP, via the enzyme NAD kinase (120). Only the cytosolic isoform of the NAD kinase is depicted in the figure. Therefore, NAD+ is a precursor for NADP. Both NAD+ and NADP+ are hybrid acceptors, and are converted to the reduced forms, NADH and NADPH. NADH transfers reducing equivalents that are derived from fuel oxidation, and, therefore, the NAD+/NADH couple is critical for energy generation. The NADPH/NADP+ redox couple is central to anabolism and antioxidant defense by donating electrons to glutathione (GSH/GSSG) and thioredoxin [Trx(SH)2/TrxSS)] systems, both of which are critical in scavenging H2O2 (generated from superoxide, O•2, by dismutation) via the enzymes GR, GPx, and thioredoxin reductase-Prx, respectively. Mitochondrial antioxidant system is mirrored by a similar scavenging mechanism in the cytosol. In these reactions, the reduced and oxidized members of the redox couples interconvert but are not consumed. Unlike these antioxidant mechanisms, catalase, which also scavenges H2O2, does not require reducing equivalents from NADPH for its function. Mitochondrial NADH/NAD+ and NADPH/NADP+ redox couples are linked by the enzyme NNT that reduces NADP+ at the expense of NADH oxidation and utilizing the mitochondrial IM proton motive force to drive this process. NNT maintains mitochondrial matrix NADPH/NADP+ pool in a reduced form, and it is a physiologically relevant source of NADPH to drive the enzymatic degradation of H2O2. The figure shows that the mitochondrial redox state of the NADH/NAD+ and NADPH/NADP+ redox couples are maintained different as the nucleotides have different metabolic roles. The NADH/NAD+ pool supports the divergent transfer of electrons from fuel substrates to both the ETC and antioxidant system via NNT, and thus is only partially reduced in comparison to NADPH/NADP+. NNT maintains the NADPH/NADP+ ratio several fold higher than the NADH/NAD+ (161). Cytosolic NADPH is regenerated via the pentose phosphate pathway, and redox reactions are catalyzed by isocitrate dehydrogenase, malic enzyme, aldehyde dehydrogenase, and methylene tetrahydropholate dehydrogenase (115). The cytosolic NADH is imported in mitochondria by redox shuttles, most commonly the M-A and glycerol 3 phosphate shuttles (G3P). GPx, glutathione peroxidase; GR, glutathione reductase; GSH, glutathione; GSSG, glutathione disulfide; H2O2, hydrogen peroxide; NNT, nicotinamide nucleotide transhydrogenase; M-A, malate-aspartate; Prx, peroxiredoxin. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars