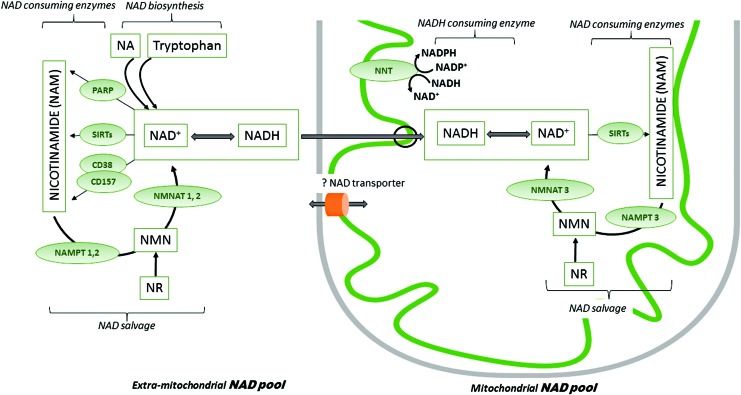
FIG. 4.
Maintenance of mitochondrial and extramitochondrial NAD pools. NAD occurs as either oxidized (NAD+) or reduced (NADH) forms. In the extramitochondrial compartment, NAD is a co-substrate for enzymes, including PARPs, sirtuins (SIRT 1, 2, 5, 6, 7), and cyclic ADP-ribose (cADPR) synthases (CD38, CD157). These enzymes decrease the extramitochondrial NAD pool by continuously degrading NAD to NAM. The major mitochondrial NAD consumers are sirtuins 3, 4, and 5. The NAD biosynthetic pathway maintains a stable NAD subcellular concentration, and it relies on precursors such as dietary NA, tryptophan, NAM, and NR. The latter two may be also administered exogenously. NAD salvage pathway recycles the NAM generated as a by-product of the NAD-consuming enzyme activities. Transformation of NAM to NMN is catalyzed by nicotinamide phosphoribosyltransferases (NAMPT 1, 2, and 3), which are rate limiting in the salvage pathway. Conversion of NMN to NAD is then catalyzed by NMN adenylyltransferases (NMNAT 1, 2, and 3). As the mitochondrial membrane is impermeable for NADH, the reducing equivalents generated in the cytosol are transferred to the mitochondria via redox shuttles. Although the NMNAT3 isoform is present in mitochondria, the mitochondrial NAD salvage pathway has not been fully elucidated. Exogenous NAD increases mitochondrial function, but a mammalian NAD transporter has yet to be found. NNT reduces NADP+ to NADPH at the expense of NADH oxidation. NA, nicotinic acid; NAM, nicotinamide; NMN, nicotinamide mononucleotide; NR, nicotinamide riboside; PARP, poly(ADP-ribose) polymerase. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars