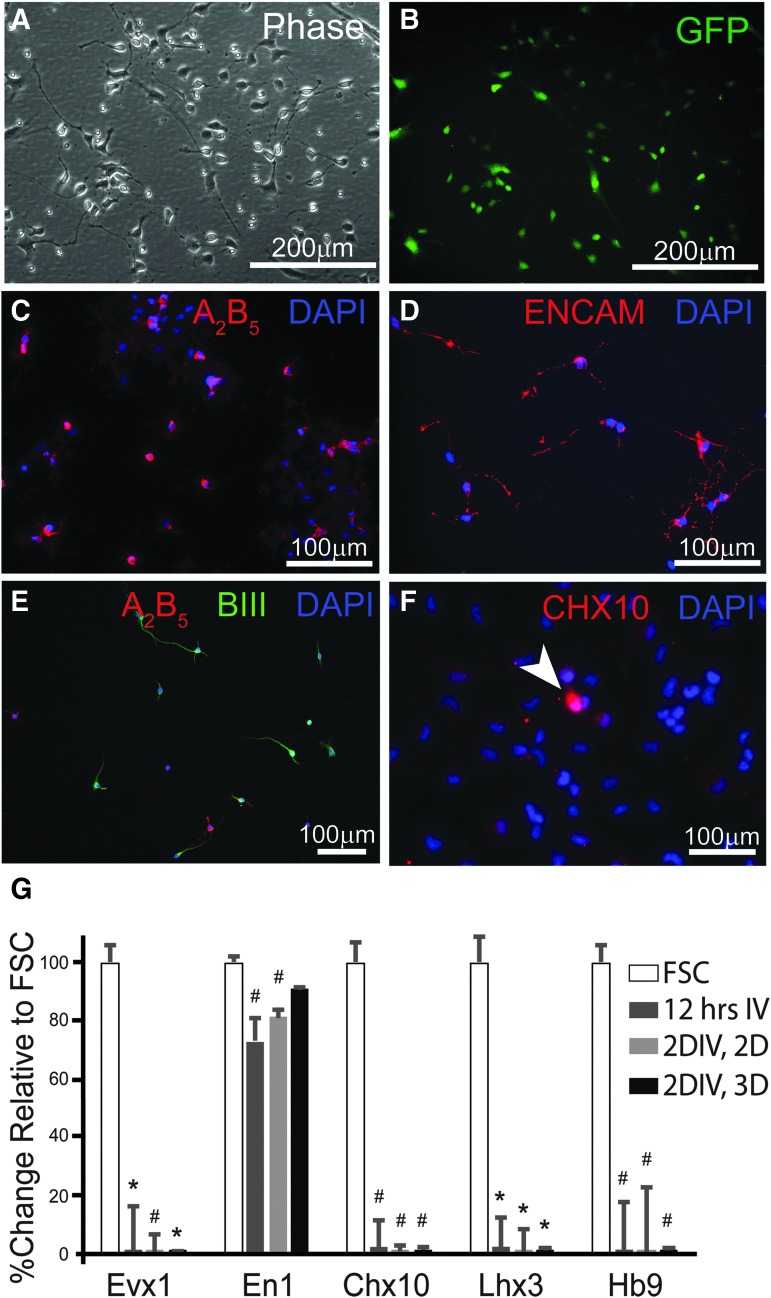
FIG. 2.
In vitro characterization of neural progenitor cells (NPCs). Phase (A) and fluorescence (B) images of NPCs show morphology typical of progenitor neurons and glia after isolation, culturing, freezing, and thawing procedures. Live-cell immunocytochemistry against A2B5 (C) and ENCAM (D) demonstrates the presence of both glial and neuronal progenitor cells, respectively, with some expression of βIII Tubulin (E). Immunocytochemistry against the transcriptional factor Chx10, a marker for V2a interneurons (INs), reveals a small population of NPCs that express the protein (F). Messenger RNA (mRNA) was isolated from NPCs thawed and cultured for 12 h on adherent, 2-dimensional flask (2D), for 2 days in vitro (2DIV, 2D) and from NPC aggregates, which are cultured in suspension or “3D” (2DIV, 3D). Quantitative real-time polymerase chain reaction analysis of these mRNA samples reveals decreased transcription of ventral IN markers (Evx1, Chx10, Lhx3, Hb9), compared with mRNA isolated from fetal spinal cord (FSC) tissue (expressed as 100%). NPCs cultured for 12 h and 2 days, but not for 2 days as aggregates have lower expression of En1 compared with mRNA in FSC samples. Scale bars are as indicated. Two-tailed Student's t-test: *p < 0.0001 and #p < 0.05 compared with FSC group.