Abstract
Ischemic heart disease is a leading cause of mortality and occurs due to coronary arterial atherosclerosis, vascular cavity stenosis and occlusion. It has previously been demonstrated that berberine treatment may ameliorate and help to prevent cardiovascular diseases due to its anti-inflammatory and anti-apoptotic effects in myocardial cells. However, the potential signaling mechanisms mediated by berberine in the progression of myocardial injury remain to be elucidated. The aim of the present study was to investigate the therapeutic effects of berberine and its potential mechanism in a mouse model of myocardial cell injury. The results revealed that berberine treatment downregulated the serum expression of inflammatory factors, including interleukin (IL)-6, tumor necrosis factor-α, IL-10 and IL-17A in mice with anoxia-reoxygenation injury. Berberine treatment also decreased myocardial cell apoptosis following anoxia-reoxygenation injury via regulating the expression of apoptosis-associated genes. Histological analysis revealed that the area, circumference fragmentation and segmentation of myocardial cells were significantly decreased by berberine treatment compared with the control group. The body weight, blood lipid levels, blood pressure and heart rate were markedly improved in mice with anoxia-reoxygenation injury following berberine treatment compared with untreated mice. The expression of p38 mitogen-activated protein kinase (MAPK) and nuclear factor (NF)-κB expression was downregulated in myocardial cells from in mice with anoxia-reoxygenation injury following berberine treatment compared with untreated mice. However, p38 MAPK overexpression ameliorated the berberine-induced decrease in NF-κB activity and expression, as well as the berberine-induced inhibition of myocardial apoptosis in myocardial cells isolated from experimental mice. In conclusion, the results of the present study indicate that berberine is able to decrease the expression of inflammatory cytokines expression and inhibit myocardial cell apoptosis via downregulating the p38 MAPK-mediated NF-κB signaling pathway. These results suggest that berberine may be an effective treatment for anoxia-reoxygenation injury.
Keywords: berberine, anoxia-reoxygenation injury, apoptosis, inflammation, p38 mitogen-activated protein kinase, nuclear factor-κB
Introduction
Cardiovascular diseases may affect the heart, brain and blood vessels and are caused by hyperlipidemia, atherosclerosis and hypertension (1,2). Cardiovascular diseases also include cerebrovascular diseases (3). Cardiovascular disease presents systemic vascular lesion or systemic vascular lesions in the performance of the heart and brains, which is the most frequent cause of death in the old population of economically developed countries (4–6). Cardiovascular disease also presents the highest mortality in the world that closely associates with metabolism disorders of the glucose and lipid metabolism (7,8). At present, anoxia-reoxygenation injury of the heart represents a serious threat to human health (9). As such, developing our understanding of the potential signaling mechanism of anoxia-reoxygenation injury is essential for the prevention and treatment of cardiovascular disease.
Anoxia-reoxygenation injury-associated coronary heart disease has high morbidity and mortality (10). Inflammation is one of the most common pathogeneses observed and serves an important role in the progression of anoxia-reoxygenation injury (11,12). It has previously been reported that berberine is able to attenuate lipopolysaccharide (LPS)-induced inflammation and extracellular matrix accumulation via regulating the nuclear factor (NF)-κB signaling pathway (13). In addition, berberine attenuates ischemia-reperfusion injury via regulating adenosine-5′-monophosphate kinase activity in both non-ischemic and ischemic areas of the rat heart (14). Furthermore, berberine protects the rat heart from ischemia/reperfusion injury via activating the Janus kinase 2/signal transducer and activator of transcription 3 signaling pathway and attenuates endoplasmic reticulum stress (15). However, the potential molecular signaling pathways mediated by berberine anoxia-reoxygenation injury remain to be elucidated.
In the present study, the potential signaling mechanisms mediated by berberine in myocardial injury were assessed. The therapeutic effects of berberine were also investigated in a mouse model of anoxia-reoxygenation injury. The results demonstrated that berberine decreased the expression of inflammatory cytokines and inhibited myocardial cell apoptosis. Together, these data indicate that berberine may have potential as a treatment for anoxia-reoxygenation injury.
Materials and methods
Ethics statement
The present study was conducted in accordance with the recommendations of the China Guide for the Care and Use of Laboratory Animals (16). Ethical approval was granted by the Ethical Committee of Heilongjiang Provincial Hospital (Harbin, China).
Animal study
A total of 20 male C57BL/6 mice (age, 8 weeks; weight range, 28–32 g) were purchased from Charles River Laboratories (Wilmington, MA, USA) and housed at 23±1°C and 50±5% humidity with a 12 h light/dark cycle and free access to diet and water. The myocardial injury model was established as previously described (17). Anoxia-reoxygenation injury mice were subsequently randomly divided into two groups (n=10 in each group). Mice were administered with berberine (10 mg/kg/day; berberine group; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) or an equal volume of PBS (control group). Cardiac function was then assessed to evaluate the efficacy of berberine.
Cell culture
Ventriculus sinister myocardial cells were isolated from mice as previously described (18) and cultured in Dulbecco's modified Eagle's medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (Thermo Fisher Scientific, Inc.) at 37°C in an atmosphere containing 5% CO2.
Endogenous overexpression of p38 mitogen-activated protein kinase (MAPK)
Myocardial cells were cultured until 90% confluence was reached, following which the medium was removed. Cells were subsequently transfected with pedue12.4-p38 mitogen-activated protein kinase (MAPK; 100 pmol; Invitrogen; Thermo Fisher Scientific, Inc.) or pedue12.4 empty vector (Control; 100 pmol; Invitrogen; Thermo Fisher Scientific, Inc.) using Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.). The p38 MAPK-overexpression myocardial cells were used to analyze the efficacy of berberine on NF-κB signal pathway 72 h following transfection.
Histological analysis
Cardiac tissues were fixed in 4% paraformaldehyde for 1 h at room temperature and embedded in paraffin for 2 h at room temperature. The sections was subjected this section to Masson's trichrome staining using a staining kit (Sigma-Aldrich; Merck KGaA) according to the manufacturer's instructions using light microscope (magnification, ×100). The infarct area was measured by Masson staining using computer-assisted planimetry (version 1.2; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
ELISA
Blood samples were obtained from experimental mice on day 30. Serum interleukin (IL)-6 (cat. no. MBS700340), tumor necrosis factor (TNF)-α (cat. no. MBS7817), IL-10 (cat. no. MBS10262) and IL-17A (cat. no. MBS26282; all Thermo Fisher Scientific, Inc.) levels in mice with anoxia-reoxygenation injury were measured using ELISA kits according to the manufacturer's protocol following treatment with berberine or PBS. Serum IL-6, TNF-α, IL-10 and IL-17A levels were measured using an enzyme microplate reader at 570 nm.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from myocardial cells using RNAzol (Thermo Fisher Scientific, Inc.), and DNase RNase-free (Sigma-Aldrich; Merck KGaA) was used to digest total RNA at 37°C for 15 min. An RNeasy kit (Thermo Fisher Scientific, Inc.) was then used to purify RNA and the concentration was adjusted 1 µg/µl. A total of 2 µg RNA was used as the template to synthesize cDNA at 37°C for 120 min, 99°C for 4 min and 4°C for 3 min. Followed by, qPCR was performed to amplify the expression of IL-6, TNF-α, IL-10, IL-17A, B-cell lymphoma (Bcl)-2, P53, p38 MAPK and NF-κB, with β-actin as a control (Table I). Thermocycling conditions were as follows: 95°C for 2 min, followed by 35 cycles of 95°C for 20 sec, 55.8°C for 30 sec and 72°C for 30 sec, followed by a final extension at 72°C for 5 min. Following RT-qPCR, agarose electrophoresis with 1% ethidium bromide was used to check the amplified PCR products. Gene expression was quantified using TaqMan Gene Expression Assays (Thermo Fisher Scientific, Inc.). Relative mRNA expression changes were calculated using the 2−ΔΔCq method (19).
Table I.
Primers for reverse transcription-quantitative polymerase chain reaction.
| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| IL-6 | TTCCATCCAGTTGCCTTCTTGG | TTCTCATTTCCACGATTTCCCAG |
| TNF-α | AGGCGGTGCTTGTTCCTC | AGGCGAGAAGATGATCTGACTGCC |
| IL-17A | ATGCACAGCCACCGCGACTT | CTTCATGACTGCCTCCAAGTAG |
| IL-10 | CAGTGCAGAAGAGTCGACTGCAAG | CGCTTGAGATCCTGAAATATA |
| Bcl-2 | 5GATGAAGTACATCCATTATAAGCTGTCACA | GCGCTCAGCCCTGTGCCACCTGTGGTCCAC |
| Bcl-xl | CCGGAATTCATGGCGA | CGCGCGGCCGCTCACTTGCTAGCAA |
| p38 MAPK | TCCCTCAGGAAGCTTGAACCTGAA | AAACCTAGGGTGTGGATGCCTCTT |
| NF-κB | TGCTTCCTGATGACGATGTA | TCCTCGGAGACTGGTAATGG |
| β-actin | CGGAGTCAACGGATTTGGTC | AGCCTTCTCCATGGTCGTGA |
IL, interleukin; TNF, tumor necrosis factor; Bcl, B-cell lymphoma; xl, extra large; MAPK, mitogen-activated protein kinase; NF, nuclear factor.
Western blotting
Myocardial cells were isolated and lysed in radioimmunoprecipitation assay buffer (M-PER for cells, T-PER for tissues; Thermo Fisher Scientific, Inc.) and homogenized at 4°C for 10 min. Protein concentration was measured using a bicinchoninic acid assay kit (Thermo Fisher Scientific, Inc.). A total of 20 µg protein was separated by 12.5% SDS-PAGE and transferred to nitrocellulose membranes. The membranes were incubated in blocking buffer (5% milk) for 2 h at room temperature, following which they were incubated with primary antibodies for 12 h at 4°C. The following primary rabbit anti-mouse antibodies were used: Bcl-2 (1:200; cat. no. ab59348), Bcl-extra large (Bcl-xl; 1:1,000; cat. no. ab178844), IL-6 (1:1,000; cat. no. ab100712), TNF-α (1:500; cat. no. ab119139), IL-10 (1:500; cat. no. ab9969), IL-17 (1:500; cat. no. ab79056), p38 MAPK (1:1,000; cat. no. ab47363), NF-κB (1:500; cat. no. ab131493) and β-actin (1:500; cat. no. ab8226; all Abcam, Cambridge, UK). Membranes were subsequently incubated with horseradish peroxidase-conjugated anti-rabbit IgG (1:5,000; cat. no. STAR36D649GA; Bio-Rad Laboratories, Inc.) for 2 h at 37°C. Bands were visualized using a Western Blotting Luminol Reagent and the band intensities were analyzed by ImageJ software 1.0 (National Institutes of Health, Bethesda, MD, USA).
TUNEL assay
Myocardial cells (1×106 cells/well) were cultured in 6-well plates and treated with p38 MAPK inhibitor SB203580 (1 mg/ml; Sigma-Aldrich; Merck KGaA) or NF-κB inhibitor PDTC (1 mg/ml; Sigma-Aldrich; Merck KGaA) for 12 h at 37°C. Apoptosis was analyzed using a TUNEL assay (DeadEnd™ Colorimetric TUNEL System; Promega Corp., Madison, WI, USA) according to the manufacturer's protocol. Myocardial tissues were incubated with the TUNEL reaction mixture for 1 h at 37°C, following which streptavidin- and DAB-bound biotin was quantified and cells were counterstained with hemalaun (Merck KGaA) and aquatex (Merck KGaA). DNA fragmentation was randomly detected by observing three fields in each tumor section using a confocal microscope at 488 nm (magnification, ×40).
Statistical analysis
Data are expressed as the mean ± standard deviation and were analyzed using Student's t-test or one-way analysis of variance followed by Tukey's post hoc test. All data were analyzed using SPSS Statistics 19.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism version 5.0 (GraphPad Software, Inc., La Jolla, CA, USA). *P<0.05 was considered to indicate a statistically significant difference.
Results
Berberine treatment downregulates the expression of inflammatory factors in mice with anoxia-reoxygenation injury
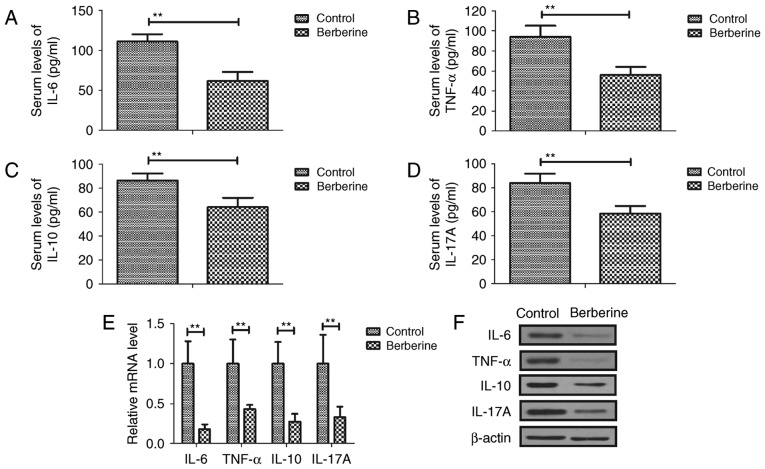
Serum IL-6, TNF-α, IL-10 and IL-17A was downregulated in the berberine group compared with the control group (Fig. 1A-D). RT-qPCR results demonstrated that IL-6, TNF-α, IL-10 and IL-17A mRNA expression was decreased in myocardial cells from the berberine group compared with the control group (Fig. 1E). The results of western blotting also revealed that IL-6, TNF-α, IL-10 and IL-17A protein expression was downregulated in myocardial cells from the berberine group compared with the control group (Fig. 1F). These results suggest that berberine treatment effectively reduced inflammation in mice with anoxia-reoxygenation injury.
Figure 1.
Berberine treatment downregulates the expression of inflammatory cytokines in mice with anoxia-reoxygenation injury. Serum (A) IL-6, (B) TNF-α, (C) IL-10 and (D) IL-17A in mice with anoxia-reoxygenation injury. IL-6, TNF-α, IL-10 and IL-17A (E) mRNA and (F) protein expression in myocardial cells from mice with anoxia-reoxygenation injury. **P<0.01. IL, interleukin; TNF, tumor necrosis factor.
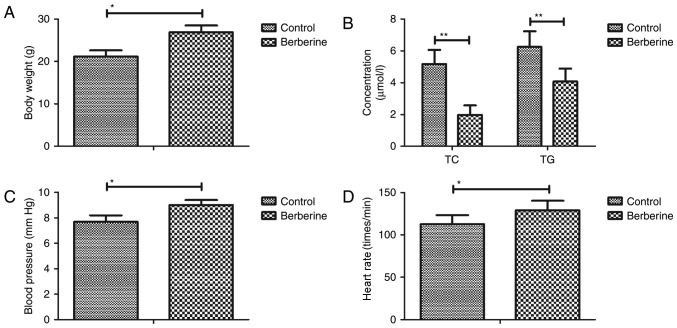
Berberine treatment improves biochemical parameters in a mouse model of anoxia-reoxygenation injury
As shown in Fig. 2A, body weight was increased in the berberine group compared with the PBS group. Furthermore, blood lipid levels were decreased in the berberine group compared with the control group (Fig. 2B). The results revealed that blood pressure and heart rate were increase in the berberine group compared with the control group (Fig. 2C and D). These results suggest that berberine treatment improves biochemical parameters in a mouse model of anoxia-reoxygenation injury.
Figure 2.
Berberine treatment improves biochemical parameters in a mouse model of anoxia-reoxygenation injury. (A) Body weight, (B) blood lipid levels, (C) blood pressure and (D) heart rate in mice with anoxia-reoxygenation injury. *P<0.05 and **P<0.01. TC, total cholesterol; TG, triglyceride.
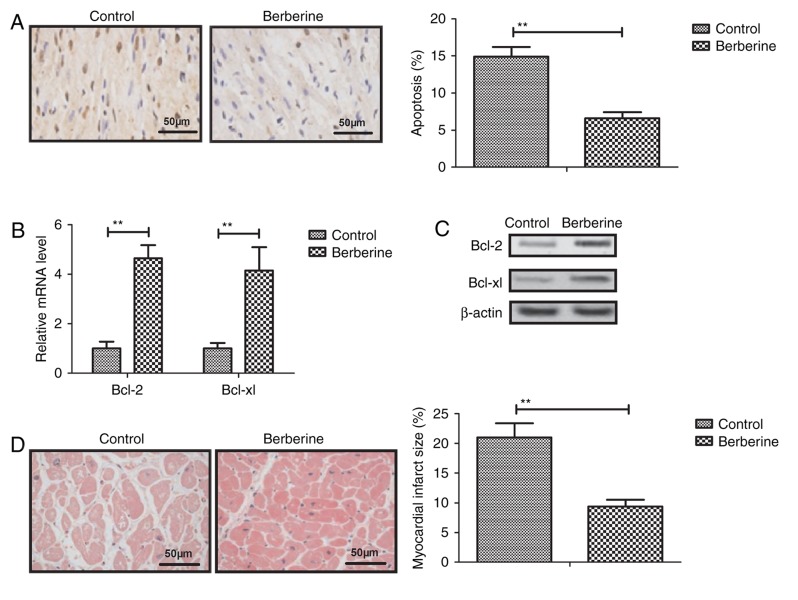
Berberine treatment reduces myocardial cell apoptosis in a mouse model of anoxia-reoxygenation injury
Berberine treatment was observed to significantly decrease myocardial apoptosis compare with the control group (Fig. 3A). The results of RT-qPCR and western blotting revealed that the expression of Bcl-2 and Bcl-xl was upregulated in myocardial cells from the berberine group compared with the control group (Fig. 3B and C). Furthermore, the myocardial injury area was significantly decreased following berberine treatment compared with the control group (Fig. 3D). These results suggest that berberine treatment significantly inhibits apoptosis anoxia-reoxygenation injury-induced apoptosis in mice.
Figure 3.
Berberine treatment reduces myocardial cell apoptosis in mice with anoxia-reoxygenation injury. (A) Berberine treatment decreases myocardial apoptosis compared with the control group determined by TUNEL. Berberine treatment upregulates Bcl-2 and Bcl-xl (B) mRNA and (C) protein expression in mouse myocardial cells. (D) Berberine treatment decreases the myocardial injury area in mice with anoxia-reoxygenation injury determined by Masson's trichrome staining. **P<0.01. Bcl, B-cell lymphoma; xl, extra large.
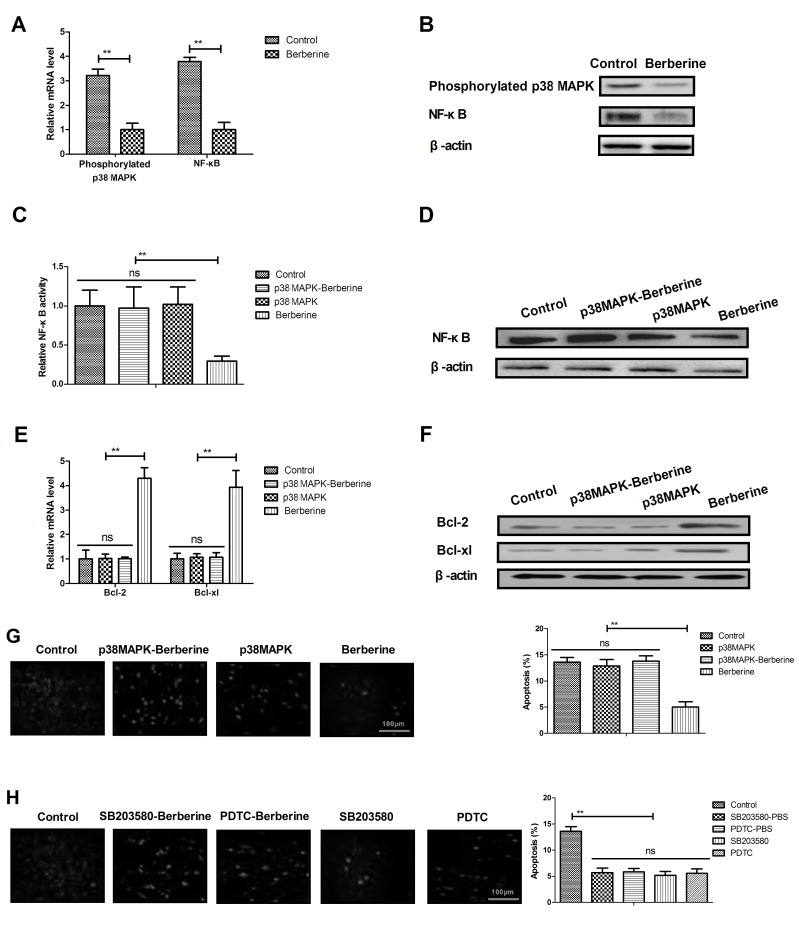
Berberine treatment improves anoxia-reoxygenation injury via the p38 MAPK-mediated NF-κB signaling pathway
As shown in Fig. 4A and B, berberine treatment significantly downregulated the expression of phosphorylated p38 MAPK and NF-κB in myocardial cells compared with the control group. It was also demonstrated that p38 MAPK overexpression effectively inhibited the berberine-induced downregulation of NF-κB activity and expression in myocardial cells isolated from experimental mice (Fig. 4C and D). p38 MAPK overexpression treatment also suppressed the berberine-induced upregulation of Bcl-2 and Bcl-xl in myocardial cells (Fig. 4E and F). It was demonstrated that p38 MAPK overexpression abolished berberine-inhibited myocardial apoptosis in myocardial cells isolated from mice (Fig. 4G). p38 MAPK or NF-κB inhibitor treatment further decreased apoptosis in myocardial cells isolated from PBS-treated mice (Fig. 4H). These results suggest that berberine treatment is able to improve anoxia-reoxygenation injury via upregulating p38 MAPK-mediated NF-κB signaling (Fig. 5).
Figure 4.
Berberine treatment improves anoxia-reoxygenation injury via the p38 MAPK-mediated NF-κB signaling pathway. Phosphorylated p38 MAPK and NF-κB (A) mRNA and (B) protein expression in myocardial cells isolated from a mouse model of anoxia-reoxygenation injury. p38 MAPK overexpression ameliorates the berberine-induced upregulation in NF-κB (C) activity and (D) expression in myocardial cells. p38 MAPK overexpression suppresses the berberine-induced overexpression of Bcl-2 and Bcl-xl (E) mRNA and (F) protein in myocardial cells. (G) Effects of p38 MAPK overexpression on Berberine-inhibited myocardial apoptosis of myocardial cells isolated from experimental mice determined by TUNEL. (H) Effects of p38 MAPK or NF-κB inhibitor on the apoptosis of myocardial cells isolated from PBS-treated experimental mice determined by TUNEL. **P<0.01. MAPK, mitogen-activated protein kinase; NF, nuclear factor; Bcl, B-cell lymphoma; xl, extra large.
Figure 5.
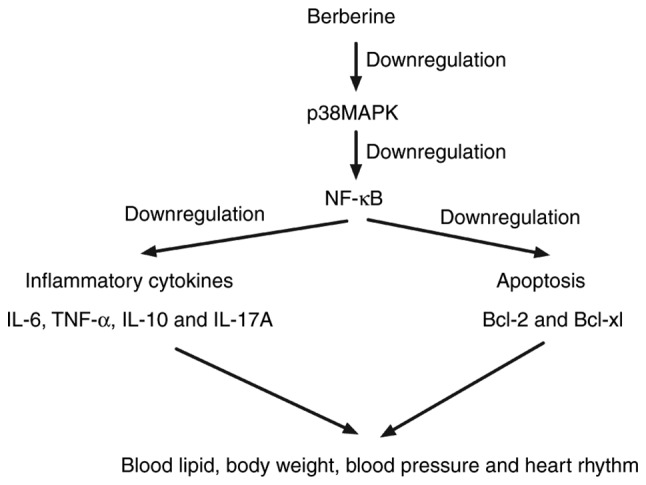
Schematic signaling pathway of berberine in myocardial cells.
Discussion
Anoxia-reoxygenation injury is the most common form of cardiovascular disease and typically occurs during myocardial infarction, cardiopulmonary bypass surgery, heart attack or heart transplantation (20,21). Inflammation serves an important role in myocardial ischemia-reperfusion injury (22,23) and it has been suggested that berberine treatment is able to attenuate cardiac dysfunction in hyperglycemic and hypercholesterolemic rats by alleviating cardiac lipid accumulation and promoting glucose transport (24). The results of the present study demonstrate that berberine treatment decreases the expression of inflammatory cytokines and improves the biochemical parameters of myocardial cells in a mouse model of anoxia-reoxygenation injury. Furthermore, it was revealed that berberine decreases myocardial cell apoptosis via upregulating Bcl-2 and Bcl-xl expression. Together, these results suggest that berberine treatment may attenuate anoxia-reoxygenation injury via the p38 MAPK-mediated NF-κB signaling pathway.
A previous study suggested that berberine attenuates adverse left ventricular remodeling and cardiac dysfunction in rats following acute myocardial infarction and that this is mediated via inhibition of the p38 MAPK pathway and activation of the pAKT pathway (25). Huang et al (26) reported that berberine treatment could alleviate cardiac ischemia/reperfusion injury by inhibiting excessive autophagy in cardiomyocytes. The results of the present study demonstrate that berberine treatment significantly decreases myocardial infarction by inhibiting myocardial cell apoptosis in a mouse model of anoxia-reoxygenation injury. Furthermore, pretreatment with berberine has been observed to protect the heart against LPS-induced myocardial dysfunction via inhibiting cardiac IκBα phosphorylation and apoptosis in mice (27). In the present study, berberine treatment attenuated the p38 MAPK-mediated NF-κB signaling pathway in a mouse model of anoxia-reoxygenation injury, suggesting that p38 MAPK may be a potential treatment target for anoxia-reoxygenation injury.
The effects of berberine on hemodynamic parameters and Ca2+ have been investigated in cardiac myocytes harvested from rats with diastolic heart failure and it was suggested that berberine may be an effective dose-dependent treatment for symptomatic relief of heart failure (28). In the present study, it was demonstrated that the protective effect of berberine in myocardial anoxia-reperfusion injury may be regulated by the p38 MAPK-mediated NF-κB signaling pathway in myocardial cells. The NF-κB pathway is associated with myocardial anoxia-reperfusion injury and may trigger the release of inflammatory cytokines (29). The results herein suggest that berberine treatment inhibits the p38 MAPK-mediated NF-κB signal pathway, which in turn downregulates the expression of inflammatory cytokines IL-6, TNF-α, IL-10 and IL-17A in mice with anoxia-reoxygenation injury.
In summary, the results of the present study indicate that berberine treatment downregulates inflammatory cytokine expression and improves biochemical parameters, including body weight, blood lipid levels, blood pressure and heart rate, in a mouse model of anoxia-reoxygenation injury. Berberine is able to regulate anoxia-reoxygenation injury via downregulating the p38 MAPK-mediated NF-κB signaling pathway, which may contribute to decreased inflammation and apoptosis in myocardial cells. These results may provide a basis for the clinical use of berberine as a therapeutic treatment for anoxia-reoxygenation injury.
Acknowledgements
Not applicable.
Funding
The present study was supported by a study on macrophage's action mechanism in post-acute myocardial infarction disposing from by the Natural Science Foundation of Heilongjiang Province (grant no. 2016-499).
Availability of data and materials
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.
Authors' contributions
XT performed the experiments. GL, KW, YX and YQ prepared and analyzed experimental data. YZ designed the study and experiments. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
Ethical approval was granted by the Ethical Committee of Heilongjiang Provincial Hospital (Harbin, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that there are no competing interests.
References
- 1.Sankari A, Martin JL, Badr M. A retrospective review of sleep-disordered breathing, hypertenstion and cardiovascular diseases in spinal cord injury patients. Spinal Cord. 2015;53:496–497. doi: 10.1038/sc.2015.16. [DOI] [PubMed] [Google Scholar]
- 2.Piepoli MF, Corra U, Abreu A, Cupples M, Davos C, Doherty P, Höfer S, Garcia-Porrero E, Rauch B, Vigorito C, et al. Challenges in secondary prevention of cardiovascular diseases: A review of the current practice. Int J Cardiol. 2015;180:114–119. doi: 10.1016/j.ijcard.2014.11.107. [DOI] [PubMed] [Google Scholar]
- 3.Kozlovskaya IL, Bulkina OS, Lopukhova VV, Chernova NA, Ivanova OV, Kolmakova TE, Karpov YA. Heat and cardiovascular diseases: A review of epidemiological surveys. Ter Arkh. 2015;87:84–90. doi: 10.17116/terarkh201587984-90. (In Russian) [DOI] [PubMed] [Google Scholar]
- 4.Klainin-Yobas P, Ng SH, Stephen PDM, Lau Y. Efficacy of psychosocial interventions on psychological outcomes among people with cardiovascular diseases: A systematic review and meta-analysis. Patient Educ Couns. 2016;99:512–521. doi: 10.1016/j.pec.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 5.Wang XQ, Pi YL, Chen PJ, Liu Y, Wang R, Li X, Chen BL, Zhu Y, Yang YJ, Niu ZB. Traditional Chinese exercise for cardiovascular diseases: Systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc. 2016;5:e002562. doi: 10.1161/JAHA.115.002562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zulli A, Smith RM, Kubatka P, Novak J, Uehara Y, Loftus H, Qaradakhi T, Pohanka M, Kobyliak N, Zagatina A, et al. Caffeine and cardiovascular diseases: Critical review of current research. Eur J Nutr. 2016;55:1331–1343. doi: 10.1007/s00394-016-1179-z. [DOI] [PubMed] [Google Scholar]
- 7.Baselet B, Rombouts C, Benotmane AM, Baatout S, Aerts A. Cardiovascular diseases related to ionizing radiation: The risk of low-dose exposure (Review) Int J Mol Med. 2016;38:1623–1641. doi: 10.3892/ijmm.2016.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fatema K, Zwar NA, Milton AH, Ali L, Rahman B. Prevalence of risk factors for cardiovascular diseases in bangladesh: A systematic review and meta-analysis. PLoS One. 2016;11:e0160180. doi: 10.1371/journal.pone.0160180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L, Xue Y, Ma H, Shi H, Wang L, Cui X. Prazosin protects myocardial cells against anoxia-reoxygenation injury via the extracellular signalregulated kinase signaling pathway. Mol Med Rep. 2018;17:2145–2152. doi: 10.3892/mmr.2017.8175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aminde LN, Veerman L. Interventions for the prevention of cardiovascular diseases: A protocol for a systematic review of economic evaluations in low-income and middle-income countries. BMJ Open. 2016;6:e013668. doi: 10.1136/bmjopen-2016-013668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Lorgeril M. Essential polyunsaturated fatty acids, inflammation, atherosclerosis and cardiovascular diseases. Subcell Biochem. 2007;42:283–297. doi: 10.1007/1-4020-5688-5_13. [DOI] [PubMed] [Google Scholar]
- 12.Candore G, Aquino A, Balistreri CR, Bulati M, Di Carlo D, Grimaldi MP, Listì F, Orlando V, Vasto S, Caruso M, et al. Inflammation, longevity, and cardiovascular diseases: Role of polymorphisms of TLR4. Ann N Y Acad Sci. 2006;1067:282–287. doi: 10.1196/annals.1354.037. [DOI] [PubMed] [Google Scholar]
- 13.Jiang Q, Liu P, Wu X, Liu W, Shen X, Lan T, Xu S, Peng J, Xie X, Huang H. Berberine attenuates lipopolysaccharide-induced extracelluar matrix accumulation and inflammation in rat mesangial cells: Involvement of NF-κB signaling pathway. Mol Cell Endocrinol. 2011;331:34–40. doi: 10.1016/j.mce.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 14.Chang W, Zhang M, Li J, Meng Z, Xiao D, Wei S, Chen L, Wang C, Hatch GM. Berberine attenuates ischemia-reperfusion injury via regulation of adenosine-5′-monophosphate kinase activity in both non-ischemic and ischemic areas of the rat heart. Cardiovasc Drugs Ther. 2012;26:467–478. doi: 10.1007/s10557-012-6422-0. [DOI] [PubMed] [Google Scholar]
- 15.Zhao GL, Yu LM, Gao WL, Duan WX, Jiang B, Liu XD, Zhang B, Liu ZH, Zhai ME, Jin ZX, et al. Berberine protects rat heart from ischemia/reperfusion injury via activating JAK2/STAT3 signaling and attenuating endoplasmic reticulum stress. Acta Pharmacol Sin. 2016;37:354–367. doi: 10.1038/aps.2015.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davey G, Wu Z. Attitudes in China toward the use of animals in laboratory research. Altern Lab Anim. 2007;35:313–316. doi: 10.1177/026119290703500305. [DOI] [PubMed] [Google Scholar]
- 17.Jong WM, Ten Cate H, Linnenbank AC, de Boer OJ, Reitsma PH, de Winter RJ, Zuurbier CJ. Reduced acute myocardial ischemia-reperfusion injury in IL-6-deficient mice employing a closed-chest model. Inflamm Res. 2016;65:489–499. doi: 10.1007/s00011-016-0931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barile L, Chimenti I, Gaetani R, Forte E, Miraldi F, Frati G, Messina E, Giacomello A. Cardiac stem cells: Isolation, expansion and experimental use for myocardial regeneration. Nat Clin Pract Cardiovasc Med. 2007;4(Suppl 1):S9–S14. doi: 10.1038/ncpcardio0738. [DOI] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Jin Y, Blikslager AT. Myosin light chain kinase mediates intestinal barrier dysfunction via occludin endocytosis during anoxia/reoxygenation injury. Am J Physiol Cell Physiol. 2016;311:C996–C1004. doi: 10.1152/ajpcell.00113.2016. [DOI] [PubMed] [Google Scholar]
- 21.Huang H, Lai S, Wan Q, Qi W, Liu J. Astragaloside IV protects cardiomyocytes from anoxia/reoxygenation injury by upregulating the expression of Hes1 protein. Can J Physiol Pharmacol. 2016;94:542–553. doi: 10.1139/cjpp-2015-0457. [DOI] [PubMed] [Google Scholar]
- 22.Xia WF, Liu Y, Zhou QS, Tang QZ, Zou HD. Comparison of the effects of propofol and midazolam on inflammation and oxidase stress in children with congenital heart disease undergoing cardiac surgery. Yonsei Med J. 2011;52:326–332. doi: 10.3349/ymj.2011.52.2.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang R, Zhang YY, Huang XR, Wu Y, Chung AC, Wu EX, Szalai AJ, Wong BC, Lau CP, Lan HY. C-reactive protein promotes cardiac fibrosis and inflammation in angiotensin II-induced hypertensive cardiac disease. Hypertension. 2010;55:953–960. doi: 10.1161/HYPERTENSIONAHA.109.140608. [DOI] [PubMed] [Google Scholar]
- 24.Dong SF, Hong Y, Liu M, Hao YZ, Yu HS, Liu Y, Sun JN. Berberine attenuates cardiac dysfunction in hyperglycemic and hypercholesterolemic rats. Eur J Pharmacol. 2011;660:368–374. doi: 10.1016/j.ejphar.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 25.Zhang YJ, Yang SH, Li MH, Iqbal J, Bourantas CV, Mi QY, Yu YH, Li JJ, Zhao SL, Tian NL, Chen SL. Berberine attenuates adverse left ventricular remodeling and cardiac dysfunction after acute myocardial infarction in rats: Role of autophagy. Clin Exp Pharmacol Physiol. 2014;41:995–1002. doi: 10.1111/1440-1681.12309. [DOI] [PubMed] [Google Scholar]
- 26.Huang Z, Han Z, Ye B, Dai Z, Shan P, Lu Z, Dai K, Wang C, Huang W. Berberine alleviates cardiac ischemia/reperfusion injury by inhibiting excessive autophagy in cardiomyocytes. Eur J Pharmacol. 2015;762:1–10. doi: 10.1016/j.ejphar.2015.05.028. [DOI] [PubMed] [Google Scholar]
- 27.Wang YY, Li HM, Wang HD, Peng XM, Wang YP, Lu DX, Qi RB, Hu CF, Jiang JW. Pretreatment with berberine and yohimbine protects against LPS-induced myocardial dysfunction via inhibition of cardiac I-[kappa]B[alpha] phosphorylation and apoptosis in mice. Shock. 2011;35:322–328. doi: 10.1097/SHK.0b013e3181facf73. [DOI] [PubMed] [Google Scholar]
- 28.Zhang XD, Ren HM, Liu L. Effects of different dose berberine on hemodynamic parameters and [Ca2+]i of cardiac myocytes of diastolic heart failure rat model. Zhongguo Zhong Yao Za Zhi. 2008;33:818–821. (In Chinese) [PubMed] [Google Scholar]
- 29.Chen H, Zhang RQ, Wei XG, Ren XM, Gao XQ. Mechanism of TLR-4/NF-κB pathway in myocardial ischemia reperfusion injury of mouse. Asian Pac J Trop Med. 2016;9:503–507. doi: 10.1016/j.apjtm.2016.03.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.