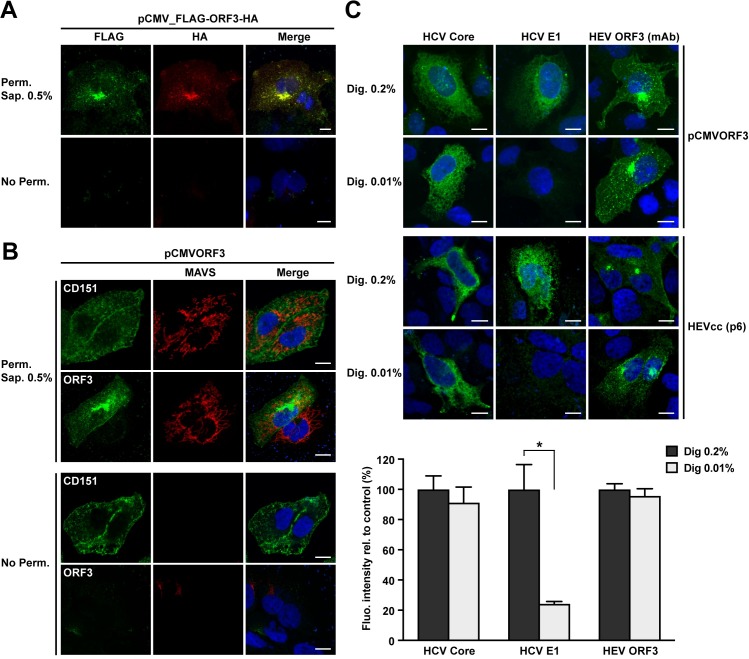
Fig 8. Membrane topology of HEV ORF3 protein.
(A) N- and C-terminal ends of HEV ORF3 protein are intracellularly exposed. S10-3 cells were transfected with pCMV_FLAG-ORF3-HA and subjected to immunfluorescence detection of HA and FLAG tags, using rabbit pAb anti-HA (Y-11) and mouse mAb anti-FLAG M2, respectively, after permeabilization with 0.5% saponin (Perm. Sap. 0.5%) or in the absence of permeabilization (No Perm.). (B) Similarly, S10-3 cells transfected with pCMVORF3 were subjected to immunofluorescence to detect the plasma membrane tetraspanin CD151 (mouse mAb 11G5a), the cytoplasmic protein MAVS (rabbit pAb anti-MAVS) or HEV ORF3 protein using mAb MRB198. Nuclei were stained by DAPI. (C) Selective membrane permeabilization. S10-3 cells were transfected with pCMVORF3 or co-transfected with pUHD15-1 and pUHD-Cp7 allowing the expression of the hepatitis C virus (HCV) core-p7 region (top panel) and cultured for 24 h. S10-3 cells were transfected with the HEV p6 infectious clone (middle panel) and cultured for 5 d. All cells were fixed and permeabilized with either 0.2% or 0.01% digitonin. Immunofluorescence detection of the cytoplasmic HCV core with mouse mAb C7-50 or the endoplasmic reticulum luminal HCV E1 glycoprotein with mouse mAb A4 served as controls for selective permeabilization of intracellular membranes. HEV ORF3 protein is detected using anti-ORF3 mAb MRB198. The lower panel shows histograms summarizing fluorescence intensities, as determined by using ImageJ software in 10 to 35 cells per condition, obtained after immunofluorescence with total (Dig. 0.2%) or selective (Dig. 0.01%) membrane permeabilization of S10-3 cells replicating the HEV p6 infectious clone. The asterisk (*) indicates statistically significant results with p<0.001. Scale bars indicate 10 μm.