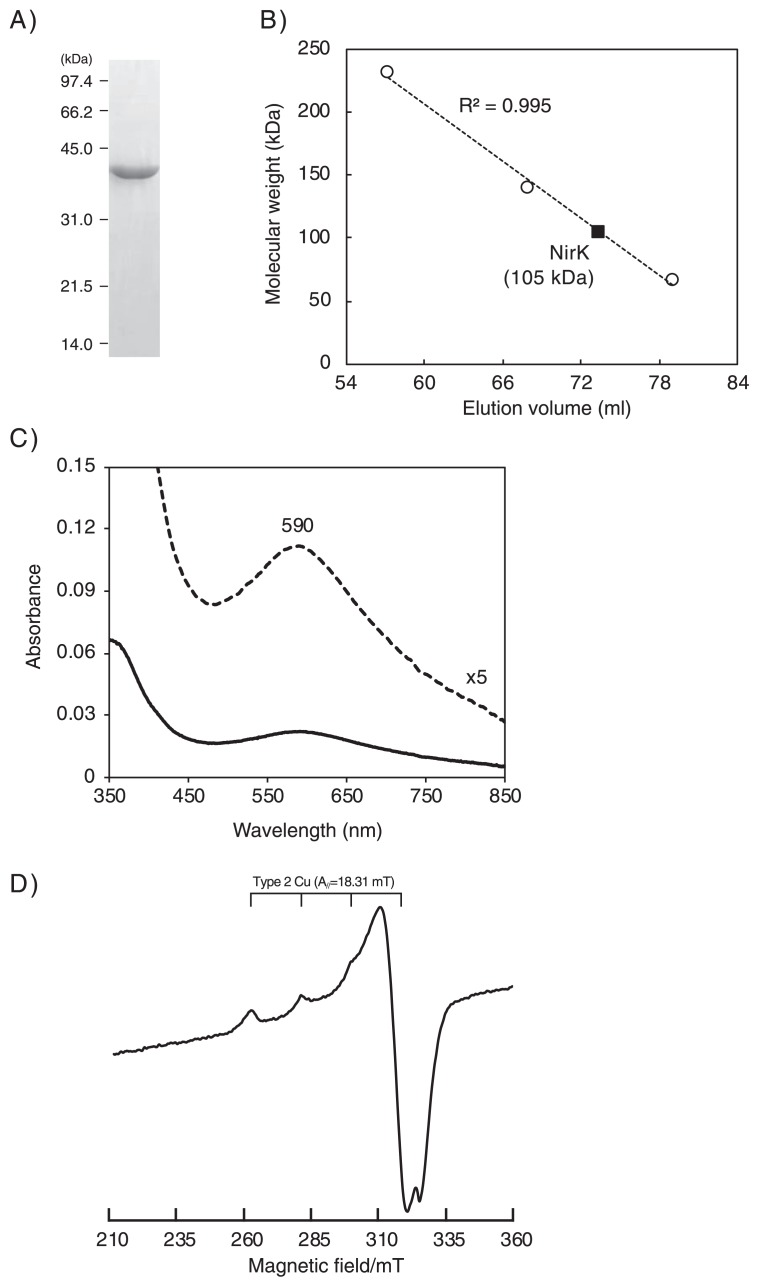
Fig. 2.
Characterization of recombinant Nitrososphaera viennensis NirK. A) SDS-PAGE of the recombinant protein purified by His-tag affinity chromatography. B) Assessment of the molecular mass of the recombinant protein by gel filtration chromatography. Catalase from bovine liver (232 kDa), lactate dehydrogenase (140 kDa), and bovine serum albumin (66 kDa) were used to prepare a standard calibration curve. C) UV-VIS absorption spectra. The measurement was performed in a 20 mM Tris buffer (pH 8) containing 300 mM NaCl at 25°C. The solid line indicates the recombinant protein (1 mL mL−1) oxidized with air. A 5×enlarged spectrum is also shown as a dashed line. D) ESR spectra. The measurement was performed using the recombinant protein (4.9 mg mL−1) at −253°C.