Abstract
Aims
Exclusion of fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) from the diet is effective in alleviating symptoms of irritable bowel syndrome (IBS) in adults. Rapid‐transit constipation (RTC) is a recently discovered subset of chronic constipation and has been linked to food intolerance. The aim of this study was to audit the effect of specific FODMAP elimination diets in children with RTC.
Methods
This was an audit of children presenting to a tertiary children's hospital surgeon with refractory chronic constipation who had rapid transit in the proximal colon on nuclear imaging; had hydrogen/methane breath tests for fructose, lactose, and/or sorbitol intolerance; and were advised to exclude positive sugar under clinical supervision. Patients filled in a questionnaire rating severity of constipation, abdominal pain, and pain on defecation with a visual analogue scale (VAS, 0 = none, 10 = high) and stool consistency for 6 months before and after dietary exclusion.
Results
In responses from 29 children (5–15 years, 21 males), 70% eliminated fructose, and 40% eliminated lactose. There was a significant reduction in the severity of constipation (VAS mean ± SEM, pre 5.8 ± 0.5 vs post 3.3 ± 0.6, P < 0.0001), abdominal pain (5.1 ± 0.6 vs 2.8 ± 0.5, P = 0.0004), pain on defecation (5.8 ± 0.6 vs 2.6 ± 0.5, P < 0.0001), and increase in stool wetness (Bristol Stool Scale pre 3.3 ± 0.3 vs post 3.9 ± 0.2, P = 0.004).
Conclusion
Children with RTC showed significant improvements in constipation and pain after excluding the sugar indicated by positive breath tests, suggesting that specific sugar‐exclusion diets may have a role in the management of RTC in children.
Keywords: exclusion diet, FODMAP, food intolerance, rapid‐transit constipation
Introduction
Constipation is common, affecting 5–30% of children, and accounts for up to 25% of referrals to gastroenterologists.1 The management of pediatric constipation in hospitals poses a significant financial burden for the health‐care system,2, 3 so effective treatment options are needed. The World Gastroenterological Association defines constipation as “a disorder characterized by persistent difficulty or seemingly incomplete defecation and/or infrequent bowel movements (once every 3‐4 days, or less) in the absence of alarm symptoms or secondary causes.”4 The majority of the cases of constipation in children are functional constipation (without an underlying organic etiology).5, 6, 7, 8, 9, 10 Defecation is a complex process, which requires structural and functional integrity of the large bowel, internal and external anal sphincter, and puborectalis muscle and intact sensory and motor nerve innervation. As the control of defecation is multifactorial and intricate, the etiology of functional constipation is poorly understood.
Chronic constipation is defined as constipation that has been present for at least 3 months.4 Chronic constipation in childhood can be refractory in spite of compliance with pharmacological and conservative management, with about one‐third of children with chronic constipation experiencing symptoms into adulthood.11 Refractory constipation is “chronic constipation not responding to maximal laxative therapy, behavioural therapy, and toilet‐training program, with duration of symptoms of >2 years.”11
Motility studies are being increasingly used to identify and define subtypes of constipation in order to manage refractory constipation more efficiently.12 Nuclear medicine gastrointestinal transit studies (NMGIT, also known as scintigraphy or nuclear transit studies) are used in children with severe constipation to determine the rate of gastrointestinal transit (from the stomach to the excretion from the anal canal) and anatomical sites of fecal retention.13 The NMGIT is a relatively noninvasive study, in which the patient ingests a meal or drink containing radioactive tracer, and the amount of radioactive tracer is measured in different areas of the gastrointestinal tract to identify areas of dysmotility. While conducting a retrospective analysis in 2011, we described a paradoxical new subset of children with intractable constipation with rapid proximal colonic transit, called rapid‐transit constipation (RTC).14 A retrospective review of patient records suggested a link with atopic tendencies. More than 40% of patients had allergic symptoms, eczema, or asthma, and 11% had a family history of an allergic condition.14 There was also a high incidence of symptoms linked to food intolerance (e.g. abdominal pain, anal fissures), suggesting that RTC could be linked to food intolerance.
Irritable bowel syndrome (IBS) is the most commonly diagnosed functional bowel disorder, affecting 7–21% of the population.15 Abdominal pain with a change in bowel habits, in the absence of organic disease, is the hallmark of IBS. Symptoms of IBS include abdominal bloating and distension, excess wind (flatulence), abdominal pain, nausea, changes in bowel habits (diarrhea, constipation, or a combination of both), and other gastrointestinal symptoms. Rome IV guidelines were recently released with specific guidance for diagnosing IBS in children.16
Food intolerances or poor absorption are common in IBS patients, and patients are recommended to follow low‐fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAP) diets.15 FODMAPs are short‐chain carbohydrates that may be poorly absorbed in the small intestine. They are easily fermented by bacteria in the colon, producing excess amounts of hydrogen and methane. Lactose, fructose, sorbitol, and inulin are FODMAPs and are restricted in a low‐FODMAP diet. A low‐FODMAP diet can reduce IBS symptoms in 70% of the patients over 3–4 weeks, and is now recommended as first‐line therapy for IBS17, 18 Diets restricting a range of FODMAPs are initiated and followed to see if symptoms cease, and then, specific sugars are reintroduced to determine which ones trigger symptoms. The aim is to identify the trigger sugars and return to a full diet with reduced amounts of the trigger. It is generally considered undesirable to stay on a low‐FODMAP diet.
The use of a low‐FODMAP diet in children is contentious, with concerns that children may lack nutrients and it could affect their bowel flora and microbiome. It is desirable to be able to identify specific trigger sugars and test a diet where these are restricted for a short time, rather than to restrict a wide range of fruits, vegetables, and grains. In a 2014 review, Darbritz et al.19 recommended lactose, fructose, or sorbitol restriction as an easily implemented therapy in children, which can result in a decrease in gastrointestinal symptoms. They supported breath tests as helpful in the evaluation of gastrointestinal symptoms in children as symptoms were resolved in two‐thirds of children, with positive results following restriction of the positive sugar. They found that patients maintained compliance with the diet restricting a particular sugar if their symptoms improved.19
Breath tests are noninvasive tests used to diagnose carbohydrate malabsorption.20, 21 Humans do not produce hydrogen or methane as a respiratory product, while bacteria in the gut ferment carbohydrates and produce hydrogen and methane that is taken up by the blood and expired in the lungs. Patients drink 20–50 g of test carbohydrate in water,20 and serial measurements of hydrogen and methane expired in the breath are obtained over 2–4 h. A positive result (10–20 ppm of hydrogen or methane above baseline on two consecutive breath measurements 15–30 min apart for 3–5 h) is a predictor of intolerance. The gold‐standard food intolerance test is food exclusion to achieve symptom improvement followed by reintroduction to prove cause and effect.22
We previously reported the effect of excluding common protein allergens (dairy, wheat, soy, eggs, nuts, and seafood) on pediatric RTC.23 Dairy and wheat were most commonly eliminated and increased frequency of stools, reduced abdominal pain, and reduced use of laxatives.23 Based on reports of specific sugars and breath tests improving symptoms,19 these were tested by our surgical group in a small group of patients with Hirchsprung disease (HSCR) with fecal incontinence with rapid colonic transit. After excluding specific sugars following breath tests, we found there was an improvement in fecal incontinence in 9 of 10 patients.24
With these positive results, one of the authors (JH) began using breath tests for patients with RTC and then recommending an exclusion diet for those with positive tests to determine if exclusion of specific sugars could affect their constipation symptoms. The aim of this study was to audit the clinic results of the effect of exclusion of the positive sugars (specifically lactose, fructose, or sorbitol) on bowel symptoms and pain in children with functional constipation and RTC, using the hypothesis that the exclusion of a specific sugar (giving a positive result in a breath test) will improve outcomes (severity of constipation, abdominal pain, pain on defecation, and stool consistency) in children with RTC.
Methods
We conducted a retrospective audit of patients who attended a surgical pediatric bowel clinic at a tertiary children's hospital between January 2015 and December 2016, with ethical approval from the Institutional Human Research Ethics Committee (31251F). Patients referred to this surgeon have severe chronic constipation that has not resolved with years of treatment by general practitioners, pediatricians, or gastroenterologists.
Breath tests
Patients were referred for breath tests to specialist pathology services (Gastrolab Gut Diagnosis, Dorevith Pathology, Heidelberg West, Victoria, Australia or Dr.Gastroenterology, St Albans, Victoria, Australia), with sample collection at specialist clinics or through home breath kits or with sample collection supervised by a specialist pediatrician for younger patients. The dose of test sugar was fixed at 10 g per patient for lactulose and sorbitol and was 1 g/kg bodyweight to a maximum of 25 g for lactose and 35 g for fructose. Samples were collected into inflatable bags to allow measurement of hydrogen and methane. Patients underwent lactulose, lactose, fructose, and sorbitol tests, and both hydrogen and methane levels were measured.
Diet restriction
The hospital received reports from the services identifying the level of sugar tolerance or intolerance. Patients with positive breath tests (20 ppm of hydrogen or methane above baseline on two consecutive breath measurements 15–30 min apart) were identified, and parents were advised to seek assistance from a health‐care professional (GP, nutritionist, dietician, pediatrician) to start a diet excluding the positive sugar. Our hospital does not have an outpatient dietician service, so parents were advised to find a clinician close to home to supervise the treatment. Parents monitored the child's diet. For additional dietary information regarding which foods to exclude for each sugar, parents were referred to Monash University's FODMAPs website (http://www.med.monash.edu/cecs/gastro/fodmap/). Diet restriction of the positive sugar was performed for 6–12 months to allow resolution of the chronic constipation, and the patients returned to the surgical clinic for follow‐up. The restriction ceased if there was no improvement in constipation. If there was improvement, parents/patients were asked to reintroduce the sugar to test causality.
Transit studies
Nuclear medicine gastrointestinal transit studies were performed using radioactive tracer in milk by the Nuclear Medicine Department, Royal Children's Hospital, Melbourne, Australia. Rapid Transit was defined as more than 25% of the tracer beyond the hepatic flexure at 6 h after ingestion and/or more than 25% of activity in the sigmoid/rectum at 24 h.14
Questionnaire
As part of their clinical treatment, a questionnaire was developed to audit the effect of food exclusion in these children. The questionnaire was created as a “Google Poll,” allowing online responses. Letters explaining the questionnaire were sent to the parents/guardians inviting them to participate, with a link to the questionnaire. The questionnaire was divided into seven sections:
Demographic information: name, age, gender, and parent's email address.
Details of the food group excluded: which sugar was excluded, how long was it excluded for, and was the exclusion complete or partial. Questions quantified consumption of the sugar before the exclusion and whether the sugar had been reintroduced to confirm its role as a trigger.
Maintenance of the exclusion diet: problems encountered by the child/parent with diet modification and if parents had sought help from a health‐care professional to implement the diet.
Laxative use before and after food exclusion: name, dose, and frequency of laxative taken.
Stool consistency before and after food exclusion was assessed using the Bristol Stool Scale (BSS).
Severity of symptoms before and after food exclusion: frequency of defecation, duration of episodes of obstipation (without defecation), incidence of abdominal pain associated with constipation, pain on defecation, anal tears, bleeding in the toilet bowl or on the toilet paper, straining, and soiling.
Quantitation of severity of constipation using a visual analogue scale (0 = does not prevent child from doing day‐to‐day activities; 5 = sometimes prevents child from doing day‐to‐day activities; 10 = often prevents child from doing daily activities most of the time, for example: playing with friends, missing school) for 6 months before and at least 6 weeks after diet exclusion. There were also questions to rate abdominal pain associated with constipation and pain on defecation on a visual analogue scale from 0 to 10 (0 = no pain, 10 = worst possible pain).
Responses to the questionnaire were entered directly into an Excel database. GraphPad Prism 7.0 was used for statistical analysis using the chi‐squared test for proportional data and the paired t‐test comparing pre versus post for VAS scores. P < 0.05 was considered significant.
Audit
Children who attended the surgical pediatric bowel clinic between January 2015 and December 2016, had positive breath tests, and were referred for exclusion diets were identified. The subset of these children who had an NMGIT study was identified. Only those who had RTC were included in this audit. We excluded children who were too young for nuclear transit studies and children with coexisting anomalies of the gastrointestinal tract, such as Hirschsprung disease (HSCR), cloacal malformations, and colostomies. The children with positive breath tests, diet exclusion, and RTC were sent a link to the online questionnaires, and data from those who replied were analyzed.
This study was approved by the RCH Human Ethics Research Committee (31251). The committee complies with international guidelines for ethical research, including the Declaration of Helsinki. The study was an audit of results of a clinical treatment, so participant consent was not necessary.
Results
A total of 104 patients (n = 104, Fig. 1) with refractory constipation who had undergone hydrogen and methane breath tests for fructose, lactose, and/or sorbitol intolerance were identified; 72 had RTC and were sent the link for the questionnaire, and 34 responded, with 2 refusing to participate and 3 replying that they had not continued with the sugar‐exclusion diet.
Figure 1.
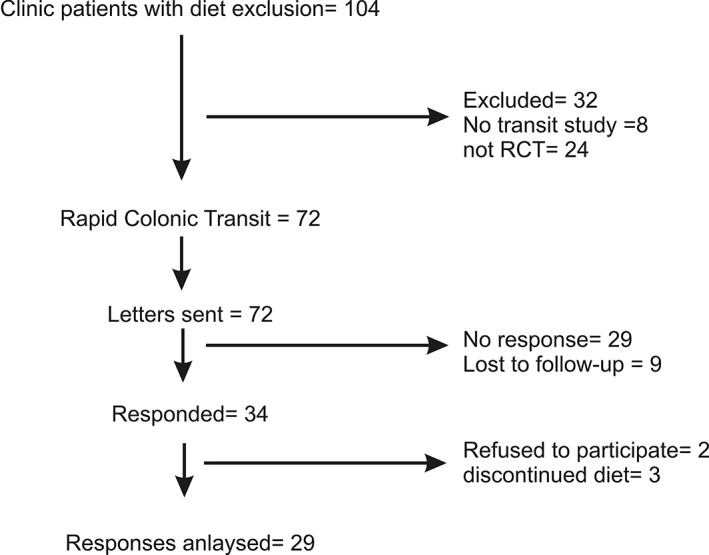
Consort diagram. Answers were received from 29 patients from 72 who were sent the questionnaires. Only patients with rapid colonic transit constipation confirmed from a nuclear medicine gastrointestinal transit study were sent questionnaires as part of their clinical treatment.
Demographic information
Of the 29 of 72 (42%) who responded, 70% were male, and the age range was 5–15 years.
Sugars excluded
Of the participants, 70% eliminated fructose, 40% lactose, and 3.3% eliminated sorbitol; 43% eliminated their positive sugar, and 57% followed a low/no FODMAP diet. Of those who eliminated fructose, 55% consumed fructose >once a day before the study, and 86% eliminated fructose completely. Of those who eliminated fructose, 46% completely reintroduced it to the child's diet to test if fructose was the trigger for constipation. Unfortunately, the parents were not asked to confirm whether the result of the trigger test was positive. Of those who eliminated lactose, 62% consumed lactose > once a day before the study, and 42% eliminated lactose completely. Of those who eliminated lactose completely, 52% reintroduced it to the child's diet to confirm lactose as the trigger for constipation.
Maintenance of exclusion diet
Of the responders, 72% (n = 21) reported that they had some problems/difficulty with implementing the diet. Of those who had difficulty with the food exclusion, 65% reported that the child “disliked the diet,” 80% of parents reported that “it took longer to prepare meals,” 40% stated that “the modified diet was unachievable with their current lifestyle,” and half (50%) of the parents felt that “food preparation was more expensive.” Common hurdles to implementing the diet included child compliance, especially at parties and in school; problems with grocery shopping; difficulties in preparing modified meals for the entire family; and difficulties monitoring the diet away from home. Understandably, dietary modification was reported to be problematic in a few of the patients who had been diagnosed with Autism Spectrum Disorder and consumed a limited variety of food before the exclusion. One of the parents reported that the food exclusion “gave some really useful insights into my son's symptoms and triggers and a few simple key changes have been made long term”. Finally, some participants stated they were allergic to some of the foods permissible on a low‐FODMAP diet. Most of the patients (84%) were supervised by a clinician (57% by a dietitian or a nutritionist, 10% by a general practitioner, and 17% by a pediatrician). Two dietitians refused to help with FODMAP exclusion.
Laxative use
Of patients, 90% (n = 26) reported the regular use of at least one laxative before initiation of the exclusion diet. Movicol (polyethylene glycol [PEG] + electrolytes) was the most commonly used laxative (65%). Movicol is an osmotic laxative that works by transporting water into the colon to soften the stool. Of the 26 who took laxatives, 19% (5/26) ceased, 42% decreased, and 14% did not change laxative use. Six patients started on a new medication, making it impossible to quantify the change in laxative usage in these patients.
Consistency of stool before and after implementation of the food exclusion
The Bristol Stool Score (1 = very hard stool, 7 = extremely loose stool) was used to quantify stool consistency. There was a statistically significant increase in stool wetness (Bristol Stool Scale pre 3.3 ± 0.3 vs post 3.9 ± 0.2, P = 0.004, Fig. 2). Before the exclusion diet, 76% of participants had a BSS < 4 (i.e. hard stools). After the exclusion diet, only 23% of participants had a BSS < 4. The proportion of patients reporting the ideal BSS score of 4 (BSS 4 = smooth, soft, and well‐formed stool) increased from 10 to 57%.
Figure 2.
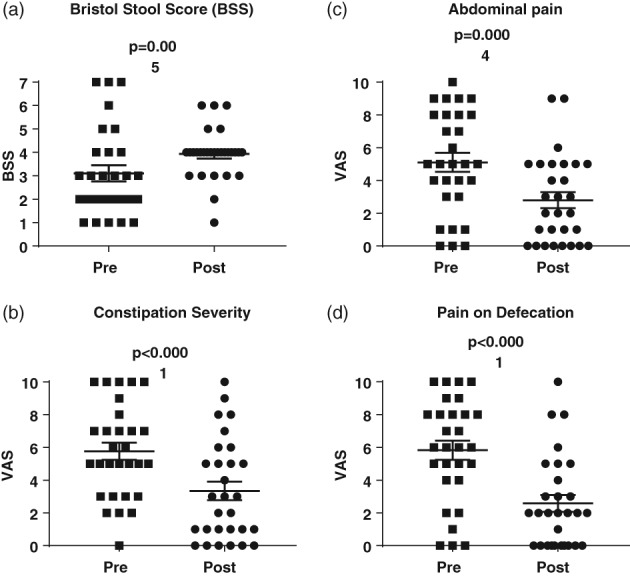
Changes in (a) stool softness, (b) constipation severity, (c) abdominal pain, and (d) pain during defecation. Stool softness was measured using the Bristol stool scale pediatric version (1 = very hard, 7 = very soft). Constipation severity, abdominal pain, and pain during defecation were measured using a visual analogue scale (VAS), with 0 = none and 10 = a lot. Participants were scored for 6 months before the diet (pre) and at least 6 weeks after the diet (post).
Severity of constipation before and after implementation of the food exclusion
The exclusion diet significantly decreased the severity of constipation, severity of abdominal pain, and pain on defecation (Table 1, Fig. 2), as measured on a VAS scale, with symptoms stopping altogether in 20–25% of respondents and reducing in 65–69%. The proportion of patients who had to strain excessively, with anal tears and blood in the toilet, reduced significantly, with the symptoms stopping in 50–75% of respondents (Table 2).
Table 1.
Effect of sugar exclusion on gastrointestinal symptoms measured with VAS score (0 = none, 10 = much)
| Number of Patients | VAS mean (SEM) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Symptom | Had symptom | Did not have | Worse | No Change | Improved ≥ 2 units | No longer present | Pre | Post | P |
| Severity of Constipation | 28 | 1 | 0 | 8 | 19 | 5 | 5.8 (0.5) | 3.3 (0.6) | <0.0001 |
| Abdominal Pain | 26 | 3 | 2 | 2 | 17 | 5 | 5.1 (0.6) | 2.8 (0.5) | <0.001 |
| Pain on defecation | 26 | 3 | 2 | 7 | 18 | 7 | 5.8 (0.6) | 2.6 (0.5) | <0.0001 |
Table 2.
Effect of sugar exclusion on gastrointestinal symptoms
| Number of Patients | Percent | P | |||
|---|---|---|---|---|---|
| Symptom | Pre | Post | Pre | Post | |
| Straining excessively | 25 | 12 | 83 | 40 | 0.001 |
| Anal tears | 16 | 4 | 53 | 13 | 0.002 |
| Daily soiling | 13 | 8 | 43 | 26 | 0.8 |
| Blood in toilet | 16 | 6 | 53 | 20 | 0.02 |
The significance of bold value shows P value <0.05.
Discussion
Exclusion of specific dietary sugars (following positive breath tests) improved the severity of constipation and associated symptoms in this selected cohort of surgical patients with chronic constipation and rapid colonic transit. Constipation resolved in 20% and reduced in more than 60% of respondents. There was statistically significant improvement in the severity of constipation, stool consistency, pain on defection, and abdominal pain. Laxative usage decreased after dietary exclusion.
The majority of parents found it difficult to implement the dietary changes to exclude one sugar. Common hurdles in implementing the exclusion diet included increased expenses and child compliance. Most parents implemented the diet modification under the supervision of a health‐care practitioner. Improvement of symptoms after dietary exclusion would be expected to positively impact the health‐related quality of life of children and their families.
The improvement in constipation, reduced laxative use, abdominal pain, and pain on defecation complements our previous study,23 where exclusion of common protein allergens (dairy, wheat, soy, eggs, nuts, seafood) improved signs and symptoms of constipation in children with RTC. Dairy and wheat were the food groups most commonly eliminated and increased frequency of stools, reduced abdominal pain, and reduced use of laxatives. While not considered at the time of that study, dairy also contains lactose, one of the common FODMAPs. That study reported similar parent‐reported difficulties with compliance to dietary modification due to increased expense and child compliance. Note that breath tests are not possible for protein intolerance.
We have also audited the effect of FODMAP elimination in a group of children with HSCR and RTC.24 Ten patients with HSCR had NMGIT studies showing RTC. Eight had lactose and/or fructose breath tests, with seven testing positive. Gastrointestinal symptoms such as fecal incontinence improved in 9 of 10 patients after FODMAP exclusion. The patients had an organic disorder (lack of enteric neurons) that was treated by surgery but also developed food intolerance and RTC that was improved by excluding the positive sugar.
Dabritz et al.19 audited 206 children with functional gastrointestinal disorders who had breath tests for lactose, fructose, and sorbitol malabsorption. They found that 46% of breath tests were positive, and the majority of those put on a restriction diet had improvement in symptoms, including pain and diarrhea. They examined cut‐off values of 10, 20, and 25 ppm and concluded that ≥20 ppm was a good cut‐off level. Some people do not produce hydrogen in breath tests as their colonic flora cannot produce the gas. Breath methane excretion provides a reliable measurement in this subgroup, improving test accuracy.19 We routinely perform methane and hydrogen breath tests.
More recently, the use of breath tests to identify patients who would benefit from dietary exclusion has become controversial,25, 26 and they are not recommended for routine practice. There is currently discussion about the value of breath tests, especially to detect small intestinal bacterial overgrowth (SIBO). Poor reproducibility of the breath test26 and recent studies in adults with IBS suggesting that dietary exclusion can improve symptoms independent of the breath test's results are raising doubts about the value of breath tests.21 Interestingly, the early rise in breath hydrogen/methane is frequently secondary to rapid gut transit.27 If sugar malabsorption is producing rapid transit, then the test cannot distinguish between these two events. In this small clinical review, all patients had rapid transit on NMGIT studies and positive breath tests, and three quarters showed improvement, including full resolution in a quarter with exclusion of the positive sugar. By design, we only examined patients with both positive breath tests and proven rapid transit.
We recognize that breath tests only provide an approximation of the underlying gastrointestinal dysfunction. However, the breath tests’ results do provide the families with some evidence about the possible cause of symptoms, which has made introduction of a special diet more rational than just trying exclusion diets empirically. Our experience shows that the exclusion diet should be followed long enough (along with laxatives) to allow recovery of the mechanical function of the dilated rectum for the chronic constipation to resolve. This may be 6–12 months. Reintroduction of the specific FODMAP sugars after the constipation resolves caused abdominal pains and/or diarrhea rather than constipation.
We implemented breath tests and specific sugar restriction following the recommendation of Darbritz et al.19 in 2014 that lactose, fructose, or sorbitol restriction can result in a decrease in gastrointestinal symptoms. They discussed the limitations of the breath test. The degree of malabsorption in a breath test depends on the dose of the FODMAP ingested (e.g. 80% of patients will malabsorb a 50‐g load of fructose, but only 10% will malabsorb 25 g, the maximum dose given in the current study), the small intestinal transit time, and inherently reduced absorptive capacity. They also report that reproducibility is poor, and malabsorption of fructose, mannitol, and sorbitol, as shown by an increase in breath hydrogen, has no relation to the induction of gastrointestinal symptoms during the test. Nonetheless, they supported breath tests and a diet restricting a particular sugar.19 More recently, the Monash group, which champions the low FODMAP diet, agrees that studies in children suggest benefit but warn that there is need for care in implementing a full FODMAP‐restriction diet due to psychological and nutritional risks.28
The dose of carbohydrate in this study was 10 g fixed for lactulose and sorbitol and 1 g/kg for lactose (maximum 25 g) and fructose (maximum 35 g). Studies in children have used lactose doses from 0.5 to 2.0 g/kg up to a maximum dose of 25–50 g, but there is no gold standard for diagnosis for lactose, fructose, or sorbitol.19 A high percentage of positive results may be due to a high test dose that is above the capacity of all patients. The amount of lactose needed to induce symptoms in someone who is lactose intolerant varies depending on numerous factors (and may be only 15 g), and the amount needed to induce symptoms in a child with lactose malabsorption (either healthy or with an FGID) is currently unknown.29 For fructose, Gomara et al. performed fructose hydrogen breath testing in children with abdominal pain using 1, 15, and 45 g doses and found that 11 of 32 (34%) had fructose malabsorption with 15 or 45 g doses.30 Of these 11, 9 (82%) had a significant improvement on a 2‐week dietitian‐recommended fructose‐restricted diet, suggesting true positives. Escobar et al. performed fructose breath testing using 1 g/kg (up to 25 g) in 222 children with abdominal pain–FGIDs and found that 121 (55%) had fructose malabsorption, with 93 (77%) improving on a low‐fructose diet.31 Dabritz et al. reviewed fructose hydrogen breath testing and found that 55 of 142 (39%) children had fructose malabsorption.19 Dabritz et al. found that 109 of 146 (75%) children with abdominal pain had sorbitol malabsorption, with most of them (27/31, 87%) improving on a sorbitol‐restricted diet, again suggesting a real condition.19 In the current study, it could be that specific carbohydrate intolerance is common in the selected patient group (surgical patients, long‐term intractable constipation, and rapid transit through the proximal colon) as removal of positive sugars from the diet allowed the symptoms to resolve.
Age is also important, with fructose malabsorption occurring more frequently in younger children, while lactose intolerance occurs in a similar proportion across all ages of children after weaning.32
More recent published evidence does not strongly support the restriction of single carbohydrates in children with FGIDs.29 Rather, as in adults with IBS, FODMAP restriction is emerging as a better clinical strategy. However many dieticians are concerned about putting children on restricted diets, and parents and children find it very difficult to impossible to achieve and maintain a full FODMAP‐restricted diet. The low‐FODMAP diet carries risks of nutritional inadequacy and possibly of fostering disordered eating and induces a potentially unfavorable gut microbiota.28 FODMAPs should be reintroduced according to tolerance during the maintenance phase of the diet. Studies of the low‐FODMAP diet in children are few but do suggest benefit28 and should be implemented with care due to the psychological and nutritional risks of a restrictive diet and a long‐term modified low‐FODMAP diet followed.
How can food intolerance produce constipation rather than diarrhea?
In a retrospective review of 520 pediatric patients who had chronic constipation and had NMGIT studies, we found a surprising number (12%) who had rapid transit through the small bowel and proximal colon but had an overall delay in transit.14 We called this paradoxical condition “rapid transit constipation.” These patients have diarrhea‐like transit in the colon while having constipation. How is this possible? We hypothesize that food intolerance increases motility in the small bowel and proximal colon, and luminal contents are rapidly expelled into the distal bowel. Stool that travels rapidly through the gut is often acidic and may cause discomfort on defecation. It may be that repeated episodes of painful diarrhea lead to secondary with‐holding behavior. Fecal retention in the distal colon is commonly seen in children with RTC.14
The significant reduction in abdominal pain, severity of constipation and pain on defecation seen with specific sugar exclusion in children with RTC is similar to results with FODMAP exclusion in IBS in adults.17, 18 This raises the intriguing possibility that RTC may be unrecognized IBS developing in these children. Symptoms are constipation with pain and with rapid transit in an NMGIT study. Constipation with pain is diagnostic of IBS‐C in adults. Rapid transit would be expected to result in diarrhea. Diarrhea with pain is diagnostic of IBS‐D. Adults with IBS may have alternating bouts of diarrhea and constipation, suggesting that rapid transit periodically overcomes the constipation.
Limitations
This study was biased and examined results only from surgical clinic patients with long‐term refractory constipation and with rapid transit in the transit study and with positive breath tests. The sample size was small (n = 29) as only 40% responded to the survey, and the results need to be verified through a study with greater power. This rate of responding, 40%, is common in surveys, but a greater response rate would provide greater representation. There may have been a response bias as less than half of the patients responded, and all were from one clinician. The questionnaire is not validated, and compliance with the diet was patient‐/parent‐reported and not verified. There were no positive and negative controls as this is an audit of clinical practices rather than an RCT.
Patients were recommended to work with a clinician but not formally referred. We felt that supervision by a dietitian would be optimal, but our children's hospital has no outpatient dietetics department. As the families would need to see a private dietitian, many decided not to pursue this option, despite the fact that we advised them that this would be the best course of action. Many families used an APP on their iPhone from Monash University giving advice on FODMAP sugars and what foods contain specific sugars. In addition, we advised families to only exclude foods containing the sugars identified by breath tests, rather than a full FODMAP diet, as is usual in adults with IBS.
Some patients reintroduced the sugar, but we did not ask for the outcome of the reintroduction of restricted sugar. As these children have chronic constipation, it is recognized that it takes many months for the rectum to regain compliance. While diet restriction for abdominal pain and diarrhea may show results within weeks, a longer period (6–12 months) is needed for the secondary symptoms of long‐term constipation to resolve.
RTC is a newly described condition,14 with diagnostic criteria based on normal transit values in adults and slow‐colonic transit values in children with chronic constipation.33 Only a few specialist centers perform NMGIT studies, and rapid transit has not been reported by other centers yet.
Future studies
Compliance with the exclusion diet might be improved by greater involvement of dietitians/nutritionists. If it is established that positive breath tests followed by dietary exclusion are successful, NMGIT studies would not be necessary, saving radiation exposure and money.
Conclusion
RTC is a new subset of constipation and requires distinct treatment from slow transit constipation. Exclusion of specific sugars significantly improved the severity of constipation, reduced abdominal pain, pain on defecation, straining, anal tears, blood in stool, and stool consistency and decreased laxative use in children with RTC. The results of this study suggest that specific FODMAP sugar‐exclusion diets have a promising role to play in the management of RTC in children.
Supporting information
Figure S1. The Questionnaire was presented as an online ‘Google Poll’ form. Families were sent the link and answered online. Data entered via the Google Poll interface went directly into an Excel table, with each patient allocated an ID number and deidentified for analysis. Families without Internet answered over the phone.
Acknowledgments
This study was not funded. Data were collected as part of clinical treatment and audited by KW as a medical student doing a research component of her degree. BS is supported by an NHMRC Senior Research Fellowship and MCRI Fellowship support 2017. This study was supported by the “State Government of Victoria Operational Infrastructural Support Program.”
Declaration of conflict of interest: JH is the treating physician for the patients, and the questionnaire was sent to parents to assess the outcomes of treatment. The questionnaire was filled in online and was not anonymous. Data were deidentified for this study. KW and BS had no relationship to patients and analyzed deidentified data. Less than half of the patients returned questionnaires.
Author contribution: KW collated and analyzed the data and wrote the first draft. CL, VP, and JW created the ‘Google Poll’ questionnaire and data collection link and collected data into an Excel file. JH cosupervised KW. He was the treating physician, organized the transit studies and breath tests and interpreted the results, advised the parents on what to exclude from their child's diet, and suggested they contact dietitians/nutritionists for support. He designed the questionnaire; organized the format with CL, VP, and JW; and sent the questionnaires out to patients. He designed the study and edited drafts. BS supervised KW, supervised data collection and analysis, supervised writing the first draft, and edited drafts. BS had full access to all data in the study and takes responsibility for the integrity of the data and accuracy of the analysis.
Funding support: State Government of Victoria. National Health and Medical Research Council, Australia. Murdoch Children's Research Institute.
References
- 1. Rasquin A, Di Lorenzo C, Forbes D et al Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology. 2006; 130: 1527–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ansari H, Ansari Z, Lim T, Hutson JM, Southwell BR. Factors relating to hospitalisation and economic burden of paediatric constipation in the state of Victoria, Australia, 2002‐2009. J. Paediatr. Child Health. 2014; 50: 993–9. [DOI] [PubMed] [Google Scholar]
- 3. Rajindrajith S, Devanarayana NM, Crispus Perera BJ, Benninga MA. Childhood constipation as an emerging public health problem. World J. Gastroenterol. 2016; 22: 6864–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burgell RE, Bhan C, Lunniss PJ, Scott SM. Fecal incontinence in men: coexistent constipation and impact of rectal hyposensitivity. Dis. Colon Rectum. 2012; 55: 18–25. [DOI] [PubMed] [Google Scholar]
- 5. Koppen IJN, Di Lorenzo C, Saps M et al Childhood constipation: finally something is moving! Expert Rev. Gastroenterol. Hepatol. 2016; 10: 141–55. [DOI] [PubMed] [Google Scholar]
- 6. Chang SH, Park KY, Kang SK et al Prevalence, clinical characteristics, and management of functional constipation at pediatric gastroenterology clinics. J. Korean Med. Sci. 2013; 28: 1356–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Steiner SA, Torres MR, Penna FJ et al Chronic functional constipation in children: adherence and factors associated with drug treatment. J. Pediatr. Gastroenterol. Nutr. 2014; 58: 598–602. [DOI] [PubMed] [Google Scholar]
- 8. Koppen IJ, Lammers LA, Benninga MA, Tabbers MM. Management of functional constipation in children: therapy in practice. Paediatr. Drugs. 2015; 17: 349–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kuizenga‐Wessel S, Benninga MA, Tabbers MM. Reporting outcome measures of functional constipation in children from 0 to 4 years of age. J. Pediatr. Gastroenterol. Nutr. 2015; 60: 446–56. [DOI] [PubMed] [Google Scholar]
- 10. Kuizenga‐Wessel S, Heckert SL, Tros W, van Etten‐Jamaludin FS, Benninga MA, Tabbers MM. Reporting on outcome measures of functional constipation in children‐A systematic review. J. Pediatr. Gastroenterol. Nutr. 2016; 62: 840–6. [DOI] [PubMed] [Google Scholar]
- 11. Youssef NN, Di Lorenzo C. Treatment options for refractory childhood constipation. Curr. Treat Options Gastroenterol. 2002; 5: 377–87. [DOI] [PubMed] [Google Scholar]
- 12. Belkind‐Gerson J, Tran K, Di Lorenzo C. Novel techniques to study colonic motor function in children. Curr. Gastroenterol. Rep. 2013; 15: 335. [DOI] [PubMed] [Google Scholar]
- 13. Mugie SM, Perez ME, Burgers R et al Colonic manometry and colonic scintigraphy as a diagnostic tool for children with severe constipation. J. Pediatr. Gastroenterol. Nutr. 2013; 57: 598–602. [DOI] [PubMed] [Google Scholar]
- 14. Yik YI, Cain TM, Tudball CF, Cook DJ, Southwell BR, Hutson JM. Nuclear transit studies of patients with intractable chronic constipation reveal a subgroup with rapid proximal colonic transit. J. Pediatr. Surg. 2011; 46: 1406–11. [DOI] [PubMed] [Google Scholar]
- 15. Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. JAMA. 2015; 313: 949–58. [DOI] [PubMed] [Google Scholar]
- 16. Drossman DA. Functional gastrointestinal disorders: history, pathophysiology, clinical features and Rome IV. Gastroenterology. 2016; 150: 1262–79.e2. [DOI] [PubMed] [Google Scholar]
- 17. Halmos EP, Power VA, Shepherd SJ, Gibson PR, Muir JG. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology. 2014; 146: 67–75.e5. [DOI] [PubMed] [Google Scholar]
- 18. Lomer MC. Review article: the aetiology, diagnosis, mechanisms and clinical evidence for food intolerance. Aliment. Pharmacol. Ther. 2015; 41: 262–75. [DOI] [PubMed] [Google Scholar]
- 19. Dabritz J, Muhlbauer M, Domagk D et al Significance of hydrogen breath tests in children with suspected carbohydrate malabsorption. BMC Pediatr. 2014; 14: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Simren M, Stotzer PO. Use and abuse of hydrogen breath tests. Gut. 2006; 55: 297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barrett JS, Gibson PR. Fructose and lactose testing. Aust. Fam. Physician. 2012; 41: 293–6. [PubMed] [Google Scholar]
- 22. Barrett JS, Gibson PR. Fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAPs) and nonallergic food intolerance: FODMAPs or food chemicals? Therap. Adv. Gastroenterol. 2012; 5: 261–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kearsey I, Hutson JM, Southwell BR. The effect of food withdrawal in children with rapid‐transit constipation. Pediatr. Surg. Int. 2016; 32: 683–9. [DOI] [PubMed] [Google Scholar]
- 24. Stathopoulos L, King SK, Southwell BR, Hutson JM. Nuclear transit study in children with chronic faecal soiling after Hirschsprung disease (HSCR) surgery has revealed a group with rapid proximal colonic treatment and possible adverse reactions to food. Pediatr. Surg. Int. 2016; 32: 773–7. [DOI] [PubMed] [Google Scholar]
- 25. Wilder‐Smith CH, Olesen SS, Materna A, Drewes AM. Editorial: rethinking predictors of response to the low FODMAP diet ‐ should we retire fructose and lactose breath‐hydrogen testing and concentrate on visceral hypersensitivity? Authors' reply. Aliment. Pharmacol. Ther. 2017; 45: 1282. [DOI] [PubMed] [Google Scholar]
- 26. Yao CK, Tuck CJ, Barrett JS, Canale KE, Philpott HL, Gibson PR. Poor reproducibility of breath hydrogen testing: Implications for its application in functional bowel disorders. United European Gastroenterol J. 2017; 5: 284–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Burgell RE, Gibson PR. The lactulose breath test in irritable bowel syndrome: is it all hot air? Dig. Dis. Sci. 2016; 61: 655–7. [DOI] [PubMed] [Google Scholar]
- 28. Hill P, Muir JG, Gibson PR. Controversies and recent developments of the low‐FODMAP Diet. Gastroenterol. Hepatol. (N Y). 2017; 13: 36–45. [PMC free article] [PubMed] [Google Scholar]
- 29. Chumpitazi BP, Shulman RJ. Dietary carbohydrates and childhood functional abdominal pain. Ann. Nutr. Metab. 2016; 68(Suppl. 1): 8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gomara RE, Halata MS, Newman LJ et al Fructose intolerance in children presenting with abdominal pain. J. Pediatr. Gastroenterol. Nutr. 2008; 47: 303–8. [DOI] [PubMed] [Google Scholar]
- 31. Escobar MA Jr, Lustig D, Pflugeisen BM et al Fructose intolerance/malabsorption and recurrent abdominal pain in children. J. Pediatr. Gastroenterol. Nutr. 2014; 58: 498–501. [DOI] [PubMed] [Google Scholar]
- 32. Jones HF, Burt E, Dowling K, Davidson G, Brooks DA, Butler RN. Effect of age on fructose malabsorption in children presenting with gastrointestinal symptoms. J. Pediatr. Gastroenterol. Nutr. 2011; 52: 581–4. [DOI] [PubMed] [Google Scholar]
- 33. Southwell BR, Clarke MC, Sutcliffe J, Hutson JM. Colonic transit studies: normal values for adults and children with comparison of radiological and scintigraphic methods. Pediatr. Surg. Int. 2009; 25: 559–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. The Questionnaire was presented as an online ‘Google Poll’ form. Families were sent the link and answered online. Data entered via the Google Poll interface went directly into an Excel table, with each patient allocated an ID number and deidentified for analysis. Families without Internet answered over the phone.


